In a successful week for the medical technology companies in the LSI Alumni community, we saw LSI Alumni secure new funds, receive regulatory approvals, and much more on the road to LSI Europe ‘25 in London (September 7-11).
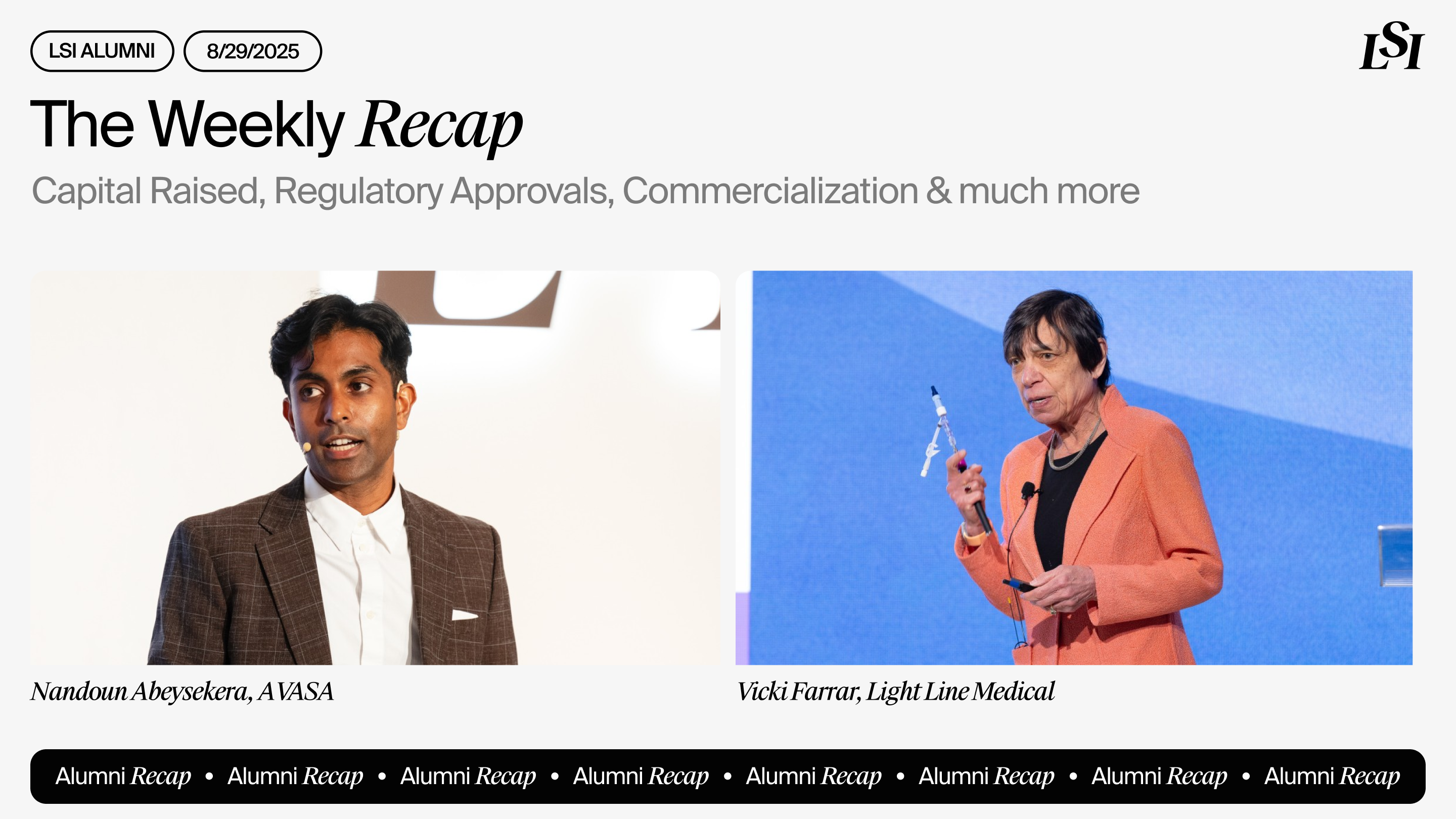
AVASA
Completed the first close of an oversubscribed Pre-Series A round led by Movac, raising NZD$4.75M to advance its category-defining AVASA Coupler device. The funding enables Avasa to complete FDA clearance and prepare for the commercial launch of its microsurgical solution for standardized arterial reconnection.
Ciliatech
Received CE mark approval for Intercil, a uveal spacer eye implant that treats glaucoma via a novel cilioscleral interpositioning approach. The implant aims to preserve anterior chamber integrity using a “no-bleb-no-cleft” method and is now set for commercial rollout across Europe with future expansion into the U.S. and China.
Facet Dynamics
Secured private equity funding to support regulatory efforts, including a pivotal, multi-center national IDE clinical study for its Restore “artificial back joint.” The funding removes regulatory risk for potential acquirers and sets a clear path to bring the novel facet joint replacement system to U.S. patients.
GT Medical Technologies
Treated its 2,000th patient with GammaTileⓇ, a bioresorbable implant that delivers localized radiation therapy immediately after brain tumor removal. The milestone highlights growing adoption across more than 200 leading cancer centers and reinforces the therapy’s impact in closing the treatment gap between surgery and radiation.
Light Line Medical
Was accepted into the FDA’s Safer Technologies Program for its light-based infection-prevention platform. The designation validates the potential impact of Light Line’s PhotoDisinfection™ System for catheter-associated infection prevention and supports a more efficient path to market.
Optheras
Raised $6M from existing shareholders to initiate its first clinical study for a laser-based treatment for superficial bladder tumors. The funds will support development of the company’s anesthesia-free, office-based procedure and builds on the recent FDA Breakthrough Device Designation for its laser and disposable fiber system.
PAVmed
Announced plans to license a novel endoscopic imaging technology from Duke University to detect and treat advanced esophageal precancer during upper endoscopy. The agreement adds a/LCI+OCT technology to its portfolio and strengthens its position in the esophageal precancer space.
Planatome
Promoted Keith Jeffcoat as President and Chief Innovation Officer and appointed Bill Fender and Matt Likens to its Executive Board. The leadership expansion strengthens Planatome’s focus on innovation and strategic growth as it advances adoption of its patented nano-polishing platform.
Plexāā
Joined Johnson & Johnson Innovation’s JLABS EMEA as a virtual member to accelerate development of BLOOM43, a wearable device that enables supraphysiological preconditioning™ before breast surgery. The milestone supports the company’s mission to reduce wound healing complications and expand its platform into additional surgical applications.
Pulse Biosciences
Published results from a first-in-human feasibility study evaluating nanosecond pulsed-field ablation (nsPFA) for benign thyroid nodules. The study showed up to 93% nodule reduction at one year on ultrasound assessment with symptom relief as early as two weeks, supporting the potential of nsPFA as a safe, effective alternative to thermal ablation or thyroidectomy.
Recor Medical
Received Japanese regulatory approval for the Paradise ultrasound renal denervation (uRDN) system, marking the first medical device cleared in Japan indicated for resistant hypertension. The approval expands global access to Recor’s CE-marked, FDA-approved technology, following strong results from the RADIANCE-HTN TRIO study.
Samay
Received a Notice of Allowance in Japan for its core acoustic resonance technology, expanding patent protection to the world’s third-largest medtech market. The milestone strengthens the company’s IP portfolio and supports potential strategic partnership opportunities in respiratory care.
SDIP Innovations
Was awarded an Industry Growth Program grant to advance its implant system for orthopedic surgeries. The co-investment supports development of SDIP Innovations’ bioresorbable bone implant technology.
The Mullings Group Companies
Announced the launch of its Strategic Initiative Service to support C-suite-driven hiring needs like build-to-buy, the build-out of a sales organization, and quality remediation efforts. The initiative, co-led by Joe Mullings and newly appointed EVP Kirk Petyo, expands TMG’s ability to execute high-volume, specialized hiring programs across medtech, healthtech, and life sciences.
Stay tuned for more updates, insights, and achievements of our LSI Alumni in The Weekly Recap, and follow our blog for the latest medical device news and advancements.

Schedule an exploratory call
Request Info17011 Beach Blvd, Suite 500 Huntington Beach, CA 92647
714-847-3540© 2026 Life Science Intelligence, Inc., All Rights Reserved. | Privacy Policy