who can make things happen
Siemens, to the entrepreneurs behind Synchron, Auris
Health, and Neotract, decision-makers convene at LSI.
real investors — but not LSI
with VCs, PEs, and CVCs who actively deploy capital — and
we create experiences they show up for, year after year.
active investors attend LSI
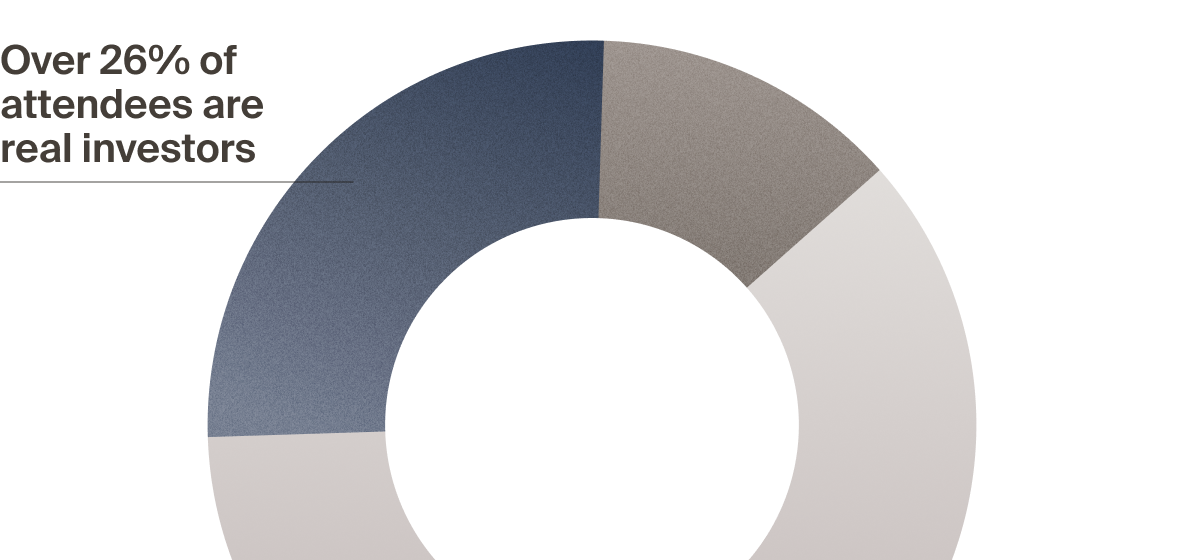
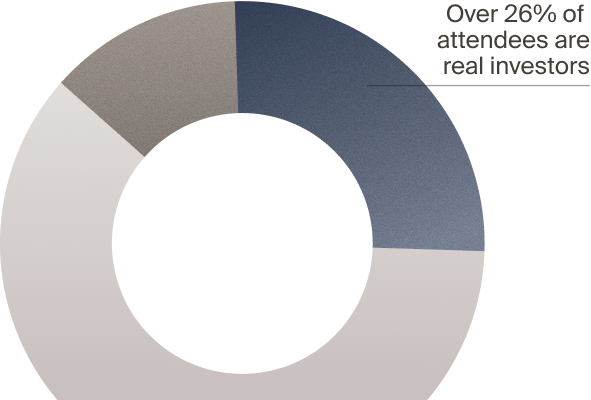
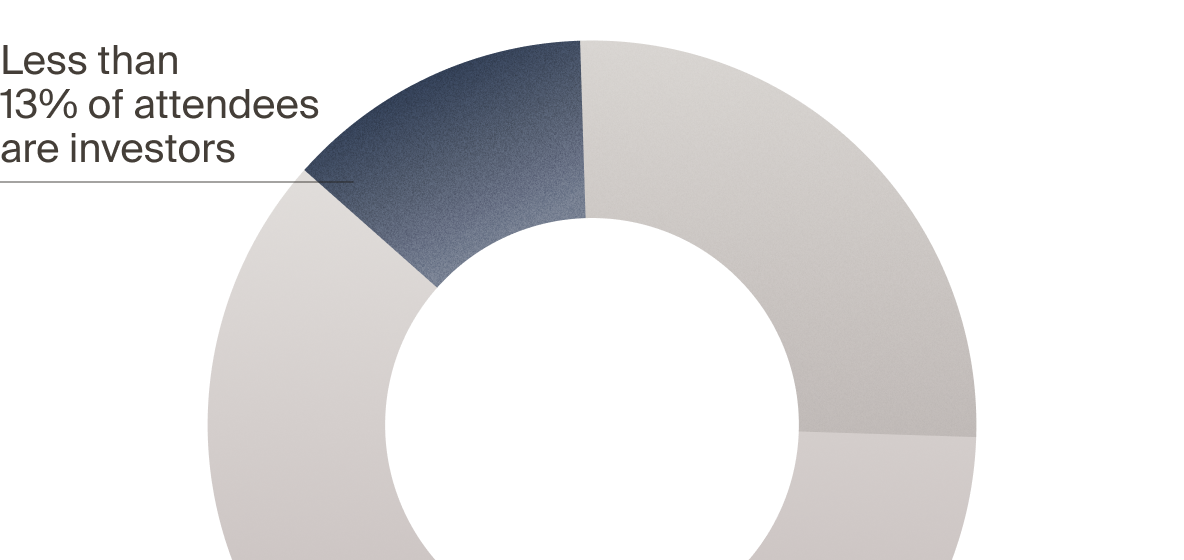
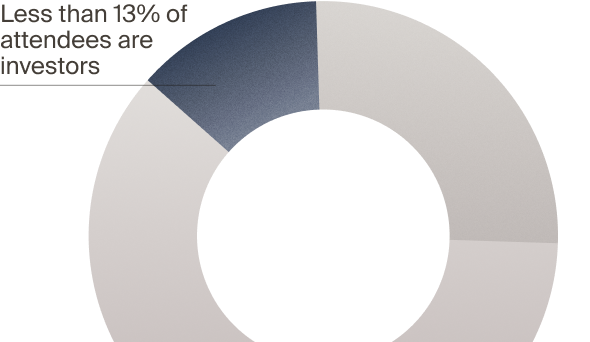
to attend LSI USA ‘26?
investment banks, global strategics, and more in London this September.
at LSI USA ‘26
building the next generation of breakthrough
medtech and healthtech companies.
-

4D Medicine Ltd is a UK-based company developing next-generation implantable devices using 4Degra®, a proprietary resorbable biomaterial based on polycarbonate urethane chemistry. Designed for use in both soft and load-bearing applications, 4Degra® overcomes key performance limitations of legacy materials like PLA and PLGA. The company’s first product, a range of interference screws for soft tissue–bone fixation, is expected to launch in the U.S. in 2026. By enabling advanced processing techniques such as 3D printing and offering tunable mechanical properties, 4D Medicine aims to transform the design and performance of resorbable medical implants. Visit website.

Phil Smith
CEO
-

Aclarion is a SaaS company leveraging MR Spectroscopy to noninvasively help physicians distinguish between painful and nonpainful spinal discs. Its cloud-based platform processes raw spectral data collected during a standard MRI, transforming it into clear metabolic biomarkers through proprietary signal processing and augmented intelligence. The resulting disc-specific metrics support more informed clinical decision-making for patients with chronic low back pain. Visit website.

Brent Ness
CEO
-

Acuamark Diagnostics is optimizing qPCR and dPCR for early cancer detection and cancer recurrence. Its approach is designed to provide highly accurate results to physicians and patients at a fraction of the cost per life saved. With market leading clinical results in stage 1-4 colorectal cancer, Acuamark is currently developing diagnostics for multiple cancers. US launch of its first products is estimated in 2026. Visit website.

Bernard Peperstraete
Cofounder & CEO
-

AdjuCor is developing reBEAT – an extravascular heart assist device for patients with advanced heart failure. reBEAT is a novel, minimally invasive, epicardial, biventricular mechanical circulatory support system designed to assist one or both ventricles without contacting the blood. Visit website.

Stephen Wildhirt
CEO
-

The SteriPlas Cold Plasma medical device by Adtec Healthcare Ltd (Twickenham, London UK) is a strong antibacterial with proven clinical efficacy for the management of chronic wounds, surgical site infections, and diabetic foot ulcers typically complicated with antimicrobial resistance (AMR) and biofilm. Where conventional therapies, such as antibiotics, fail to destroy superbugs protected within biofilm leading to a worsening infection, the SteriPlas delivers a quick, painless and fast-acting solution with faster treatment times than standard of care and reduced hospital stays. Visit website.

Mary McGovern
CEO
-
Ajemiri Neurotech is developing a portable, dry-sensor EEG platform combined with machine learning to detect electrophysiological biomarkers of Alzheimer’s disease. Our approach enables low-cost, non-invasive brain health screening suitable for memory clinics, primary care, and clinical trial recruitment.

Joshua Ajemiri
Founder & CEO
-

Altoida is developing the NeuroMarker Platform to transform how cognitive disorders are detected, monitored, and managed. The platform enables continuous, real-time monitoring of cognitive function, allowing for early identification of subtle changes. By providing actionable insights, it supports clinicians in personalizing treatment plans and tracking patient progress over time. Altoida’s approach also enhances patient engagement by offering clear visibility into cognitive health, empowering individuals to actively participate in their care. Visit website.

Marc Jones
CEO
-

Amber Implants is developing the VCFix Spinal System, a vertebral body augmentation system to improve pain control and stabilize the spine in patients suffering from vertebral compression fractures (VCFs). The company intends to improve the treatment of painful spinal fractures through improvements in mechanical performance, providing controlled restoration via more patient-specific implant customization, and offering superior stabilization that reduces the biomechanical load on the vertebral body and the spine. Visit website.

Mohammad Ahmadi
Co-founder & CTO
-

Amplifi Vascular is developing the Amplifi™ Vein Dilation System, a proprietary blood pump technology designed to enlarge veins and improve outcomes for patients requiring arteriovenous fistula (AVF) surgery. The system is intended to increase AVF eligibility, accelerate maturation, reduce failure rates, and extend primary patency. By addressing key challenges in dialysis access, Amplifi aims to improve treatment outcomes for patients with end-stage renal disease. Visit website.

Sean Morris
President & CEO
-

Anaut is a Tokyo-based technology company developing AI precision mapping technology to enhance surgical outcomes and reduce complications. Its flagship product, Surgical Vision EUREKA, uses advanced algorithms and deep learning to provide real-time mapping of the surgical field. The technology aims to support surgeons in navigating complex anatomy with greater accuracy. Visit website.

Roy Bartel-Taverner, MD
Chief Medical Officer (Interim)
-

AngioInsight is developing AngioAI+, an AI-driven solution for real-time, non-invasive assessment of stenosis and Fractional Flow Reserve (FFR). The technology provides rapid and accurate data on flow dynamics and anatomy, aiding in the detection of microvascular disease. By integrating automated AI diagnostics into the workflow, AngioAI+ enhances precision and efficiency in cardiovascular assessment. Visit website.

Scott Burger
CEO
-

Treating gastrointestinal disease with disruptive, vapor-based endoscopic ablation therapy. Visit website.

Lloyd Mencinger
President & CEO
-

Arsenal Medical develops innovative biomaterials to solve challenging and underserved medical problems. Our lead products target neurovascular and trauma conditions providing control of blood flow to tumors and bleeding conditions, such as hemorrhage. Visit website.

Upma Sharma
President & CEO
-

ART MEDICAL develops the smART+ Platform to address malnutrition and reduce the risk of ventilator-associated pneumonia in intensive care units. The platform offers features that enhance staff productivity and enable precise, data-driven care. Clinically proven to reduce ICU stays and ventilation duration by 3.3 days, smART+ achieves up to 100% feeding efficiency through real-time reflux detection, dynamic nutritional compensation, and continuous tube positioning monitoring. Visit website.

Ori Braun
CEO
-

AthenaDiax produces a portable ECG device and software currently in use within the Italian clinical market to diagnose patient heart health. Our product performs cardiac diagnostics that monitor and detect heart issues for early treatment of our patients. Our next stage is to enter the US market to provide proactive self-service heart monitoring directly to consumers. Visit website.

David Schwietz
COO & CIO
-

Axena Health is dedicated to improving the lives of women with pelvic floor disorders. The company's Leva Pelvic Health system helps women train and strengthen their pelvic floor muscles from their homes to improve the management of stress, mixed, and mild-to-moderate urinary incontinence and chronic fecal incontinence. Visit website.

Randy Pritchard
CEO
-

Axorus develops a contact lens to restore sight for blind patients suffering from dry AMD (age-related macular degeneration) and Retinitis Pigmentosa, based on patented photoacoustic technology from Boston University. Visit website.

Jean-Damien Louise
CEO
-

AyrFlo is advancing a new category of physiological monitoring called Breathonics, which captures the mechanics of airflow through the body’s natural airway system. Unlike traditional tools that rely on indirect proxies such as pulse oximetry or CO₂, AyrFlo’s technology measures directional airflow velocity continuously and non-invasively. This approach enables new clinical applications in areas such as post-surgical care and respiratory disease. AyrFlo aims to establish a new standard for monitoring respiratory function with real-time, source-based measurement. Visit website.

Guelay Bilen-Rosas
CEO
-

Berlin Heals is developing the C-MIC system, an implantable electroceutical device that delivers a constant, but minimal, electrical direct current that mimics the physiological electrical currents produced by the heart. The device is a near-curative treatment for patients with heart failure that propagates the reverse remodeling of cardiac muscle tissue to restore heart function and size, thereby reducing the symptoms of heart failure and improving quality of life. Visit website.

John Brumfield
CEO
-

Biobot Surgical's mission is to revolutionize urologic oncology care with the Mona Lisa platform, which enables robotic-assisted biopsy and treatment of prostate cancer Visit website.

Adrian Whitford
President
-

BIOCAPTIVA is a leading innovator in liquid biopsy technology, transforming diagnostic workflows through its proprietary msX™ platform. This breakthrough technology enables direct extraction of high-quality circulating cell-free DNA (cfDNA) and RNA from biological fluids, including whole blood without the need for chemical reagents. The msX platform addresses critical market needs for cost efficiency, scalability, and multi-omics compatibility, making it a cornerstone in advancing precision medicine. Visit website.

Jeremy Wheeler
CEO
-

Novel medical devices to enhance the reliability and safety of orthopedics procedures. Visit website.

Kambiz Behzadi
President
-

Brain4care offers an FDA-cleared and non-invasive Intracranial Pressure (ICP) dynamics monitoring technology, eliminating the invasiveness of the current gold standard and unlocking its applicability for patients with brain damage, beyond the ICU. By breaking down the barriers to accessibility and expanding its utility by using an AI platform, the company aims to make ICP monitoring a routine and essential part of patient care anytime and anywhere. Visit website.

Carlos Bremer
President North American Division
-

BrainSpace is developing a solution to simplify critical care in neurocritical patients. BrainSpace is committed to improving the way brain pressure is managed in patients recovering from stroke, traumatic brain injury, and neurosurgery. Currently patients requiring intracranial pressure management must be meticulously monitored and measured hourly by a nurse. The company has developed an automated monitoring solution as an alternative to the antiquated drainage monitoring relying on a manometer-based drain and blood pressure monitor that eliminates the hassle for patients and providers while improving the quality of data being captured for researchers and physicians. Visit website.

Caitlin Morse
CEO
-

Byonyks is reinventing peritoneal dialysis so that patients can maintain a lifestyle where they can receive treatment at home, continue to earn a living, avoid the exhaustion of hemodialysis, protect their residual kidney function, and improve their heart health. 52% of dialysis patients die because of heart failure. Visit website.

Farrukh Usman
Founder and CEO
-

CAIRA is developing software for advanced joint replacement. The software utilizes radar technology to improve navigation during manual and robotic surgery. The company's first product is a navigation system for use during knee replacement surgery. Visit website.

Jon Greenwald
CEO
-

Calla Lily Clinical Care is a women's health focused company which has developed the proprietary Callavid® platform for intravaginal drug delivery. Initial target indications are in IVF and miscarriage prevention, followed by delivery of live biotherapeutics (LBPs) to reduce antibiotic usage which contributes to antimicrobial resistance (AMR). Further use-cases include effective sample collection to facilitate the next generation of biomarker-based diagnostic tests for endometriosis and a wide range of other conditions. Visit website.

Lara Zibners
Co-Founder & Chairman
-

CAPS Medical (Israel) has developed a proprietary PlasmaSure technology that uses non thermal plasma to selectively target tumors through minimally invasive procedures. The PlasmaSure system uses existing minimally invasive tools and robotic systems, targeting a $100B TAM. CAPS effectively eliminates cancerous cells while preserving healthy tissue, allowing treatments to move from the operating room into a doctor's clinic. The technology is clinically tested and applicable to wide range of tumors. Visit website.

Ilan Uchitel
CEO
-

This approach has been validated in a confirmatory/pivotal study, on patient suffering from symptomatic Severe Aortic Stenosis (sSAS), and not recommanded for immediate TAVR or SAVR ore refusing such interventions. As next steps, Cardiawave will support studies to demonstrate the value of Valvosoft on patients suffering from Moderate Aortic Stenosis. Visit website.

Carine Schorochoff
CEO
-

CDX is a compact portable extracorporeal bypass pump for partial cardio-pulmonary support, adaptable to multiple circuit configurations including oxygenators. CDX's technology is an innovative, light-weight support system with multiple power options. The CDX pump platform is a disposable cartridge combined with a re-useable controller and stator. The entire system is less than 6 pounds in weight and can be primed and ready to support in less than 2 minutes. visit website

Gopal K. Chopra
Co-CEO
-

Cellviva is a clinical-stage platform company developing novel Anti-VEGF therapies for ophthalmology and dermatology, targeting conditions that affect millions globally. Backed by a leadership team with deep industry expertise, Cellviva's approach is supported by strong IP protection, trade secrets, and a fast-track FDA approval pathway. Strategic partnerships in development and manufacturing help de-risk capital investment and accelerate time to revenue, projected by 2028. Visit website.

Rahul Chopra
CEO
-

CergenX is developing an AI-powered solution to identify newborns at highest risk of brain injury. Its FDA-designated breakthrough device, CergenX Wave, delivers expert-informed brain function assessments at the point of care in just 15 minutes. The technology aims to support timely intervention and improve outcomes in neonatal neuroprotective care. Visit website.

Jason Mowles
CEO
-

ChamberTech is a UK-based MedTech startup pioneering the first hybrid endocardial/epicardial pulsed field ablation (PFA) system for atrial fibrillation. Our catheter platform integrates novel electrode design, selective energy delivery, and dual-access ablation to achieve more durable lesions with enhanced safety. Supported by a £1.5M Innovate UK Biomedical Catalyst grant, ChamberTech is advancing toward early feasibility human studies following successful preclinical validation.

Richard Chambers
Founder & CEO
-

Charco Neurotech has an innovative medical device that is improving quality of life for People with Parkinson’s disease, the world’s fastest growing neurological condition. CUE1+ is an easy to use non-invasive, wearable device that has been shown to deliver clinically meaningful improvement in the motor symptoms associated with Parkinson’s disease, restoring mobility and quality of life to the patient, with the potential to significantly reduce care costs. Visit website.

Andrew Mullen
CEO
-

Circular Genomics is delivering precision medicine in neurology and mental health using novel, brain-enriched circular RNA (circRNA) biomarkers. We are delivering disruptive diagnostic, prognostic and therapy selection tools that radically transform the standard of care in diseases such as Alzheimer’s and depression, to elevate the standard of care, transform lives rna restore hope. Visit website.

Paul Sargeant, PhD
President and Chief Executive Officer, Member of Board of Directors
-

Clairity develops AI-driven tools to enhance breast cancer screening and risk assessment. Its platform utilizes deep learning models to analyze mammographic images, aiming to improve early detection, reduce racial and ethnic biases, and personalize care plans. The technology is grounded in decades of research led by Dr. Connie Lehman at institutions such as Mass General Brigham and MIT. Clairity's approach seeks to transform mammography from a one-size-fits-all model to a precision medicine framework. Visit website.
-small.jpg)
Jeff Luber
CEO
-

Connected Consumables integrates connectivity into infusion consumables to create a cloud to vessel end-to-end digital solution. The system enables safer, faster and automated therapies with a superior patient experience. First clinical applications are targeted at both center-based and home oncological therapy. Visit website.

Nora Herzog
CEO
-

Coramaze Technologies is developing an fast end effective solution for the treatment of tricuspid regurgitation. The TriPair system is deigned to be anchored atraumatically to the right atrium. The device is intended to be easily placed in a procedure taking less than twenty minutes, without the need for anesthesia or TEE. TriPair is agnostic of valve morphology and can be retrieved if necessary Visit website.
-small.jpg)
Jochen Reinöhl
CEO
-

CorTec develops and manufactures implantable neurotechnology devices, components, and sub-assemblies for medical devices, clinical studies, and research applications. With over a decade of experience, the company supports partners from early concept through to commercialization. CorTec aims to be the development and manufacturing partner of choice for implantable technologies, enabling the next generation of medical innovation. Visit website.

Martin Schuettler
CTO
-

Corticale develops next-generation brain-computer interface (BCI) technologies to treat motor and speech disorders linked to brain-related diseases. Its implant platform enables simultaneous interaction with thousands of neurons across broad brain regions, offering highly detailed signals to AI-based decoders. This level of neural communication aims to improve decoding performance, stability, and treatment efficacy beyond the limitations of current BCI systems. Visit website.

Fabio Boi
CTO
-

CorWave develops and manufactures innovative implantable blood pumps based on a breakthrough membrane technology. Visit website.

Louis de Lillers
CEO
-

Cresilon is a biotechnology company that develops, manufactures, and markets hemostatic medical devices utilizing the company’s proprietary hydrogel technology. The company’s plant-based technology has revolutionized the current standard by stopping bleeding in seconds. The company’s current and future product lines target trauma care, biosurgery, and animal health. Visit website.

Joe Landolina
CEO and Co-Founder
-

Crimson Scientific is building a modernized ECG with the goal of making cardiac diagnostics fast, accurate, and simple. Their device is a conformable and reusable weighted pad that simply lays on the patient’s chest without the need for adhesives or wires. The result is sent directly to an existing ECG machine for printing and archiving. Visit website.

Michael Cetta
CEO
-

Crocevia Medical is developing a solution for unmet needs in the neurovascular space. Visit website.

Tamir Nahmias
CEO
-

Cryosa has developed a novel, minimally invasive, obstructive sleep apnea (OSA) treatment that does not require a mask or an implant. Cryosa targets a root cause of OSA, excess fatty tissue. By “CoolsculptingTM” the tissue, it reduces volume and opens the airway to improve disease severity and symptoms. Cryosa was founded in 2018 and is located in Arden Hills, MN. Visit website.

Laura Stoltenberg
President and CEO
-

CustomSurg solves complex bone fractures. The technology, originally developed at Harvard Medical School and ETH Zurich, allows surgeons to treat complex bone fractures based on a clinically validated algorithm, making surgeries simpler, better, and faster. The device has recently been awarded breakthrough designation by the FDA. Visit website.

Valentin Splett
Co-CEO
-

Cypher Medical has developed a novel technology for visualizing red blood cell sedimentation. By integrating this technology into a waste container, it automatically separates red blood cells from other fluids, providing a fast and efficient method for identifying red blood cell sedimentation during surgery or childbirth. This patented solution can be applied to various biological fluid collection products. Cypher Medical is based in Texas and has partnered with an internationally recognized tech-development organization to advance its product development. Visit website.

Chris Carew
CEO
-

DeepSight Technology develops advanced medical imaging solutions using proprietary sensing technology to enhance diagnostic ultrasound capabilities. The company’s NeedleVue Technology is designed to improve ultrasound-assisted needle guidance, addressing challenges such as complex calibration, reduced sensitivity, and angle dependence. By integrating innovative sensor technology with interventional devices, NeedleVue enables real-time verification of device placement, aiming to reduce procedural uncertainty and streamline interventional workflows. DeepSight Technology operates from the San Francisco Bay Area and Clayton, Missouri, with a focus on expanding the range and effectiveness of diagnostic ultrasound. Visit website.

Diku Mandavia
Chief Medical Officer
-

Description coming soon. Visit website.

Steven Mickelsen
CEO
-

Dialybrid develops hybrid vascular grafts based on proprietary Silkothane®, a novel composite material joining the biocompatibility of silk fibroin and the elasticity of polyurethane. The first product in the pipeline is SAG (Silkothane® Arteriovenous Graft), a semi-degradable arteriovenous graft for use as hemodialysis vascular access, offering the short-term usability of synthetic grafts and the long-term performances of native fistulae. The product is currently ready to be tested in a first-in-human study. Visit website.

Stefania Riboldi
Project Coordinator
-
Dxcover’s proprietary PANAROMIC™ Platform shines light on the difficulty of early cancer diagnosis. By utilizing a multiomic spectral analysis (MOSA-Dx™) approach to detect cancer, the platform can detect the presence of disease with minute volumes of sample, with a turnaround time of one day. This technology goes beyond other liquid biopsy methods by harnessing the power of AI to capture the promise of the multiome, allowing early-stage detection of a range of solid tumors. Dxcover's unique AI algorithms are built on data; over 9000 patients and 250,000 spectra ensure robust diagnostic performance that can be tuned for high sensitivity or specificity. The test result is designed to be a valuable tool for clinicians to make rapid and appropriate patient management decisions. Dxcover’s proprietary technology is patented globally. Visit website.

Matthew Baker
CEO
-

EBAMed develops innovative solutions for non-invasive and painless treatment of heart arrhythmias by using radiotherapy. The company's technology consists of hardware and software components that enable non-invasive heart motion imaging that synchronizes in real-time with a therapeutic proton beam. Visit website.

Marina Izzo
CEO
-

Echopoint Medical is developing a family of optical-fiber based sensors which integrate into microcatheters to guide and improve outcomes during interventional cardiology procedures. The company's iKor platform translates ultrasonic signals to measure blood pressure and flow to more accurately assess, diagnose and treat patients with coronary artery disease and coronary microvascular dysfunction. Visit website.

Antony Odell
CEO
-

EFA developed RevDx™, a revolutionary, CE-approved, mobile, and fully automated diagnostic system for the point of care, including primary care physicians, home-care, emergency, and remote locations. A very easy-to-use process from a finger prick to results in a few minutes. The first application is the Complete Blood Count test and more to follow Visit website.

Yoel Ezra
CEO & Founder
-

ElectraDx has developed the first lab-accurate, ultra-low-cost community ad home based diagnostic platform using proprietary intelligent electronic strip technology. Our device delivers results from a simple fingerstick in under10 minutes, connects securely to healthcare systems, and supports a broad test menu covering over 70% of common lab tests. With an initial focus on cardiac markers, we are shifting diagnostics from hospitals into every home to enable earlier intervention and better health outcomes. Visit website.

Veronique Ameye
CEO
-

Embolic debris is released into a patient’s bloodstream during any transcatheter heart procedure such as TAVR. This debris causes ischemic brain damage that is associated with stroke and cognitive decline, as well as damage to major organs such as kidneys. Emboline, Inc. is a privately held medical technology company based in Santa Cruz, CA that has developed the Emboliner® Embolic Protection Catheter, which is a capture-and-remove embolic filter providing full brain and body protection to reduce stroke, cognitive decline and kidney damage caused by embolic debris released in a patient’s bloodstream during such procedures. Visit website.

Scott Russell
CEO
-

Enzyre is developing the ENZYSYSTEM, a near-patient blood coagulation testing platform that combines a painless sampling device, single-use microfluidic cartridges, and a cloud-connected processor to deliver rapid results for remote therapy management. Visit website.

Waander Van Heerde
CSO
-
-small.png)
EP Solutions is improving clinical outcomes in cardiac resynchronization therapy cases through the use of noninvasive cardiac mapping technology. The company's Amycard 01C is an electrophysiology workstation that records body surface electrograms to create optimized 3D heart models that merge ECG data with anatomical data obtained from medical imaging. EP Solutions' technology helps physicians accurately place CRT leads during implantation and optimize devices during follow-up visits. Visit website.

Marcin Golebicki
CEO
-

Epion Therapeutics is developing a minimally-invasive treatment that can bring early intervention to patients with corneal degeneration. Keratoconus is a common type of corneal ectasia that results in corneal thinning that progresses to the cornea taking on a conical shape that causes functional vision loss. Epion’s EpiSmart solution combines the delivery of a high concentration of riboflavin into the stroma with a UVA light device to optimize cross-linking and enhance therapeutic effectiveness. Visit website.

Michael Webb
President & CEO
-

Certalign is an industry changing, patented ‘one size fits all’ needle guide and stabilizer for ultrasound guided procedures (intravenous cannulation, nerve blocks, biopsies among others), the only device of its kind designed to accept ultrasound probes and needles of all sizes/types from most common manufacturers. Certalign will transform this space enabling crucial treatment to be provided safely, effectively and in a timely manner, delivering better patient outcomes in a cost-effective way for healthcare providers.

Preshan Jeevaratnam
Chief Medical Officer
-

Flomics Biotech is developing a revolutionary liquid biopsy test that is capable of detecting multiple types of cancer with high accuracy and with the convenience of a standard blood extraction. By combining Genomics and Artificial Intelligence we detect molecular patterns associated with the disease that are capable of directing diagnosis even before the first symptoms appear. By detecting cancer in early stages, we make sure everybody gets the best odds to win the battle against this devastating disease. Visit website.

João Curado
CEO
-

Fluid Biomed is developing the world’s first bioabsorbable neurovascular stent to cure brain aneurysms Visit website.

John Wong
CEO
-

Galena Innovations is developing the solution to spontaneous preterm birth. Unlike traditional interventions that focus on compressing the cervix, the Hannah Cervical Cup™ employs a revolutionary stretching technique. This innovative approach increases cervical stiffness and remodels collagen, addressing spontaneous preterm birth in a unique and effective manner. Visit website.

Ashley Crafton
CEO & Founder
-

Inspired by the ankle joint, which is less prone to arthritis despite carrying more weight than the hip, MaltaHip features three unidirectional articulations made from clinically accepted materials that closely mimic real joint motion, offering greater range of motion and joint stability than existing hip replacement implants. Cadaveric surgeries by leading orthopaedic surgeons from Malta and the UK showed that MaltaHip delivers excellent stability and can accommodate extreme joint angles without dislocating. MaltaHip's design also addresses the problem of wear, with laboratory testing at EndoLab® in Germany simulating over five years of walking, confirming that MaltaHip wears at least 75% less than conventional implants, translating into the potential for the implant to last 50+ years; double that of current hip implants available on the market. Visit website.

Simon Mifsud
CEO
-

GID BIO is a late-clinical-stage medical device company pioneering a point-of-care autologous cell therapy platform for treating chronic degenerative diseases, starting with knee osteoarthritis (OA). This condition affects over 30 million Americans. By harnessing each patient's own stromal vascular cells- not stem cells- GID offers a same day outpatient procedure that has shown in its phase 3 FDA IDE trial, statistical significance versus placebo, to reduce pain by 70% and improve function by 90% at one year. GID's patented disposable device isolates and concentrates over 25+ million stromal cells from a small sample of a patient's adipose tissue (fat). Visit website.

David Springer
Executive Chairman
-

GlucoModicum is developing SOFIO, a needle-free, low-cost CGM with proven proprietary technology and performance, designed for the underserved 300M people with diabetes. The company is 15 months from mass-scale commercial readiness and is currently raising €8M in equity with commitments already in place, supported by €25M in non-dilutive funding from the European Investment Bank. Visit website.

Jokke Maki
Managing Director
-

Over 2 billion people suffer from chronic foot, knee, hip, or back pain—without knowing the problem starts below the ankle. Most treatments ignore the root cause: talotarsal joint instability. GraMedica fixes it at the source with a 20-minute, motion-preserving procedure that delivers 10X better outcomes—creating a scalable path to mobility, relief, and market growth. Visit website.

Joe Monroe
CEO
-

GrOwnValve, a Charité and German Heart Center Berlin spin-off, is developing a breakthrough heart valve technology that uses a customized 3D-printed Mould to shape autologous tissue intra-operatively into a functioning heart valve within the operation room. The valves are mounted on a novel resorbable stent, folded for catheter-based delivery, and implanted minimally invasively. This approach fuses advanced manufacturing, smart biomaterials, and interventional cardiology to create the first durable heart valve solution for both children and adults. Visit website.

Boris Schmitt
CEO
-

GuideAI Health automates the early detection of peripheral vascular disease in CT scans through advanced AI models developed by clinicians and AI/ML experts from IBM Watson Health. The platform addresses the 90% miss rate in current practice by rapidly identifying and reporting subtle disease markers, enabling early intervention before life altering complications develop through disease-to-treatment precision. Visit website.

Raj Shah
CEO
-

Gulf Medical Technologies is a medical device company developing Klens, a technology that maintains clear visualization during robotic and minimally invasive surgeries. The system prevents lens obstruction from debris, fog, and fluids, enabling surgeons to operate without workflow interruptions. In collaboration with Mayo Clinic, the company is advancing Klens with computer vision and artificial intelligence to support integration into existing surgical platforms. Visit website.

Ahmad Nabeel
Founder & CEO
-

H2 Global Group is a pioneer in using molecular hydrogen in the fields of healthcare (including biotech and medtech), wellness, beauty, and veterinary medicine with patented solution for Alzheimer disease. Visit website.
-small.jpg)
David Marsalek
President
-

Hadleigh Health Technologies is a medical device company focused on improving newborn and pediatric healthcare. Based in Sausalito, California, the company designs and manufactures innovative health technologies for global markets under ISO 13485 certification. Since 2015, Hadleigh has partnered with manufacturing and fulfillment teams across the U.S., Asia, Latin America, and Europe to deliver high-quality solutions for early-life care. Visit website.

Robert Miros
CEO
-

Happitech has developed a CE-marked, smartphone-based method for effective remote monitoring of patients. The company's core competencies in artificial intelligence (AI) integration and photoplethysmography (PPG) enable effective and accurate measurement of heart rate and atrial fibrillation. Visit website.

Yosef Safi Harb
Founder and CEO
-

HapticHeart Solutions is advancing a wearable haptic feedback platform—featuring a glove and armband—that restores tactile sensation in catheter-based, minimally invasive, and robotic procedures. By converting physical and physiological signals into real-time touch and force feedback, the system aims to enhance surgical precision, shorten learning curves, and improve patient outcomes. Currently at Technology Readiness Level 6, the prototype has demonstrated clinical utility in electrophysiology and is being expanded for use in robotic surgery. Visit website.

Christopher Lee
Co-Founder and CEO
-

HealthMosaic™ is one of the first vertically integrated healthcare platforms, functioning as a clinical AI marketplace that unifies medical devices, real-time data, and interoperable systems. It delivers full-stack AI solutions with best-in-class healthcare data, models, infrastructure, and deployment to improve outcomes across the care continuum.

Justin Ramsaran
CEO
-

The Yacoub Heart Valve: A unique, tissue-engineered valve that promises to revolutionise heart valve replacement with no compromises. Using intricate scaffold technology that transforms in-situ into a living heart valve, using the body’s own healing processes to regenerate as nature intended. Restoring full life expectancy and in kids, adapting and growing. Visit website.

Francis White
CEO
-

Helico’s minimally invasive endoscopic platform uses soft robotics and AI to augment clinicians in delivering the next generation of hearing care. Its steerable balloon otoendoscope guides tools through the ear canal to hard-to-reach anatomical targets to support precision treatment delivery to the middle and inner ear. Visit website.

Lukas Lindenroth
Co-founder and CEO
-

HEPTA Medical develops a minimally invasive, safe and efficient treatment for early-stage lung cancer. HEPTA Medical's device is a flexible microwave ablation probe which can be introduced through the patient's airway to provide local thermal ablation of the cancer. The company's probe employs a proprietary patented temperature sensor enabling accurate monitoring of tissue temperature in depth during the procedure and provides real-time feedback on the ablation zone to the clinician. Visit website.

Thomas Bancel
CEO
-

Hy2Care’s CartRevive® hydrogel implant is an in-situ forming hydrogel that fills cartilage defects with precision, creating a supportive matrix for natural tissue healing. It adheres to the damaged area, protects surrounding tissue, and guides healing while new cartilage forms. Visit website.

Sanna Severins
COO
-

Image Navigation develops technologies to support precision and training in dental care. Its Image Guided Implantology (IGI) system uses CT scans and presurgical planning to assist with accurate dental implant placement. The company also offers DentSim, an augmented reality simulation platform designed to enhance dental education and training. Visit website.

Lawrence Obstfeld
CEO
-

Implican is developing a medical device designed to improve outcomes in colorectal surgery by reducing the risk of anastomotic leakage. The Implican Anastomotic Device uses a controlled compression technique with two rings to securely connect bowel segments, promoting primary wound healing. Preclinical studies have demonstrated stable tissue connections, supporting the company’s goal of enhancing recovery for colorectal cancer patients following surgical resection. Visit website.

Ivo Kooijiman
CEO
-

InFlo Medical's MOBYBOX is a portable extracorporeal membrane oxygenation (ECMO) system designed for in-hospital cardiorespiratory support with prehospital deployment capabilities. The system provides continuous extracorporeal life support across care environments, enabling patient transport and treatment without circuit interruption. Visit website.

Nicholas Williams
CEO
-

InkSpace Imaging pioneers a transformative printed electronics platform, initially prioritizing advanced MRI receive coils. These lightweight and flexible coils not only elevate diagnostic imaging, revealing the once invisible, but also expedite scans, enhance patient experiences, reduce the need for sedation, and minimize procedures. Compatible with existing MRI systems and healthcare reimbursement structures, the company's FDA-cleared innovation represents a next-generation approach to accessible, high-quality diagnostics. Visit website.
-

INNITIUS develops a medical device that combines intravaginal hardware to measure cervical tissue consistency with shear elastic waves and machine learning algorithms. The device aims to provide diagnostics for multiple women’s health conditions related to childbirth timing, with dedicated algorithms for each specific indication Visit website.
-small.jpg)
Ruben Molina
CEO & Co-Founder
-

Innotive Diagnostics is developing a novel bacterial infection diagnostic system to revolutionise the diagnosis of urinary tract infections (UTIs). Delivering a 99% reduction in time to result at the point of care using its diagnostic system vs. gold standard laboratory methods. Innotive’s technology is based on novel single-cell analysis that could be applicable across a range of bacterial infections. Visit website.
-small.jpg)
James Mainwaring
Chief Commercial & Operating Officer
-

Inossia is developing a platform technology focused on reducing repeat fractures in patients receiving bone cement for fracture repair. The company's lead product, V-Flex, is a bone cement for vertebral augmentation that hardens at a significantly lower temperature compared to competing bone cements. The soft cement is ideal for osteoporotic bones to adjacent fracture risk without compromising on mechanical support. Visit website.

Malin Nilsson
CEO
-

Interlinked is a medical technology company developing ReLink, a breakaway connector designed to reduce complications from intravenous (IV) line dislodgement. The device preserves catheter placement in situations that would normally result in removal, while also preventing fluid leakage. This approach aims to improve safety and reduce interruptions in IV therapy. Visit website.

Katarina Hedbeck
CEO & Co-Founder
-

IQ Endoscopes develops single-use flexible endoscopes designed to address challenges associated with traditional reusable scopes. Their devices aim to reduce the risks of cross-contamination and eliminate the need for complex reprocessing. The company’s first-generation gastroscope, the Q Vision G-100, has received both FDA 510(k) clearance and UKCA approval. IQ Endoscopes is working towards expanding its product range to include additional gastrointestinal endoscopes by 2025. Visit website.

Matt Ginn
CEO
-

Juniper Biomedical is developing a precision neuromodulation micro-implant to treat pelvic health conditions. Visit website.

David Constantine
CEO
-

LEM Surgical is focused on creating and providing the next generation of robotic surgical systems. The company's flagship product, the Dynamis System, is a multi-arm robotic surgical system that incorporates novel navigation capabilities and has an open architecture for handling concentric tools. Dynamis is intended to be a platform technology for orthopedic and neurosurgical applications. Visit website.

Christopher Prentice
CCO
-

Leucadia Therapeutics has developed Arethusta, an implantable medical device designed to restore cerebrospinal fluid (CSF) clearance and treat the root cause of early-stage Alzheimer’s disease and related neurodegenerative conditions. Implanted by a neurosurgeon in a minimally invasive, 30-minute outpatient procedure, Arethusta passively removes metabolite-laden CSF from the base of the brain—helping to reverse the memory and cognitive decline characteristic of early Alzheimer’s. Visit website.

Doug Ethell, PhD.
Founder & CEO
-
-small.png)
Life Seal Vascular is developing an aneurysm sac management technology designed to reduce the incidence of Type II endoleaks following Endovascular Aneurysm Repair (EVAR). Type II endoleaks are the leading cause of reintervention after EVAR, and the Life Seal device is compatible with all EVAR systems. Currently in the pre-clinical stage, the company anticipates achieving First In Man (FIM) in Q2 of 2025. Visit website.

Matt Thompson, MD, FRCS
CEO
-

LIMIS is developing PerfusiX Imaging, a dye-free surgical visualization system using Laser Speckle Contrast Imaging (LSCI) to assess tissue perfusion in real time with any existing laparoscopy tower. The company is preparing for FDA 510(k) and CE-MDR submission and is actively raising funding to accelerate certification, production and strategic integration with major MIS & RAS OEMs. Visit website.

Nils de Koff
Head of Product & Operations
-

Linio Biotech is developing a first-in-kind regenerative medical device for natural tissue augmentation, leveraging its proprietary Tience® technology. Our goal is to create a paradigm shift in aesthetic medicine by replacing temporary fillers and invasive procedures with a safe, injectable product thatregenerates soft tissue — naturally and sustainably. Visit website.

Karita Reijonsaari
CEO
-

Liposphere is a biomedical startup developing biocompatible, water-based nanomaterials designed to achieve ultralow friction in the human body. Spun out from the Weizmann Institute of Science, the company’s technology leverages the Hydration Lubrication Mechanism to create lubricious coatings for biological and artificial surfaces. Its platform, Coat by Liposphere, supports both pure lubrication and drug delivery applications, with investigational products like CCoat aimed at protecting cartilage surfaces in joints. Visit website.

Sabrina Jahn
Co-Founder & CBO
-

Liquet Medical's mission is to transform the way vascular disease is treated. The Versus Catheter features dual tips that enables treatment of both lungs, in addition to real-time pressure monitoring sensors which allows physicians to optimize the amount of drug delivered while limiting potential side effects. Visit website.

John Schindler
CEO
-

Locate Bio is working to solve some of the most complex degenerative and infectious diseases that affect the muscular skeletal system. Locate utilises Programmed Drug Release to re-align the bioavailability of existing drugs with the underlying biological need. This bio-optimisation step has demonstrated break through improvements in performance in preclinical testing. Locate Bio is reshaping rhBMP-2 perspectives with its unique, delayed presentation of rhBMP-2. It’s lead product, LDGraft is being evaluated in a human clinical study. In a large animal model, the product has demonstrated equivalent efficacy to the gold standard, but more importantly, an excellent safety profile, with none of the adverse events reported by other studies. Visit website.

John von Benecke
CEO
-

Pi is a multimodal software platform for cancer imaging and diagnostics. It is CE certified and deployed across Europe and the UK to support the analysis of MRI scans for prostate cancer, the most common cancer in men. Visit website.

Antony Rix
CEO and Co-Founder
-

Lupin Dental has created an end-to-end solution to deliver beautiful and clinically excellent smiles to patients using planning software and a supervised automated robotic treatment platform. Lupin Dental’s robotic solution enables every dentist to achieve best-in-class minimally invasive tooth preparation for porcelain laminate veneers repeatably and accurately, thereby restoring the patients’ teeth following best in class aesthetic and clinical practice. Visit website.

Paul Roberts
CEO
-

LuSeed's DOME is a minimally invasive device for treating bifurcation and sidewall brain aneurysms simply and effectively. The device utilizes a braided mesh design that makes it position-agnostic within the aneurysm. Another benefit of the DOME device is its design, which minimizes protrusion to the parent artery, thereby mitigating the need for dual anticoagulation therapy. Visit website.

Nitzan Hirsh
Co-Founder and CEO
-

OdonAssist(tm) is a soft, inflatable device for assisting the vaginal birth of newborns, the first entirely new method to deliver newborns in hundreds of years, offering a gentle option for assisted vaginal birth designed to be safe for mothers and babies. Visit website.

Gary Cohen
CEO and Co-Founder
-

May Health is developing a minimally invasive treatment for polycystic ovary syndrome (PCOS), a leading cause of female infertility due to ovulatory dysfunction. Current treatment options—including drug therapy, surgical ovarian drilling, and IVF—carry risks such as multiple pregnancies, invasiveness, and high costs. May Health’s approach aims to replicate the effectiveness of ovarian drilling while reducing procedural risk and invasiveness. The goal is to enable more natural conception pathways for women with PCOS. Visit website.

Colby Holtshouse
CEO
-

Medyria has developed the PyCath, a microcatheter for full Coronary physiology targeted to therapy optimization. The PyCath awaits FDA clearance and will be launched in US in 2026. Visit website.

Mauro Sette
CEO
-

Methinks vision is to provide universal and timely medical assistance to enable life-saving treatments worldwide. The company is initially focused on stroke, the second most common cause of death and a major cause of disability globally. Its AI software, Methinks Stroke Suite, is capable of assisting in stroke triage and providing decision support for life-saving treatment using the simplest medical imaging (non-contrast CT), with the potential to optimize stroke triage and reduce time to treatment. Visit website.

Pau Rodriguez
CEO
-

MicroSteer develops a robotic platform, enabling controlled navigation and manipulation of standard endoscopic instruments. The technology is designed to integrate with existing endoscopes to facilitate en-bloc resection of large or flat gastrointestinal polyps through precise, semi-automated tissue dissection. Visit website.

Eyal Ben-Esti
CEO
-

Microsure’s MUSA robot enables microsurgeons to perform sub-millimeter anastomosis for patients who - for example - had to undergo breast amputation or suffer from lymphedema. Visit website.

Iwan van Vijfeijken
CEO
-

myBiometry is a platform technology to remotely monitor and manage patients with chronic conditions. The initial application for the company's technology will be asthma. Through a combination of machine learning, a proprietary single-use sensor and measurement device, the company is collecting and monitoring biomarker data to understand treatment efficacy, adherence, and risk of costly asthma attacks. Visit website.

Bryan Nolan
CEO
-

Nano4Imaging has developed an magnetic resonance (MR) compatible guidewire to transfer select cardiovascular procedures from the cathlab to the MRI suite. Initially focused on developing their solutions for congenital heart diseases, the company plans to expand their technology into interventional procedures, such as angioplasty. Visit website.

Rudolf Schulze Vohren
CEO
-

Nanoflex Robotics is developing next-generation, remote robotic solutions to enhance access to life-saving procedures. The company's robotic system uses advanced magnetic technology and ultra-flexible endoluminal tools to enable faster and easier navigation through tortuous blood vessels. Nanoflex's initial target application aims to shorten the time to treatment for acute ischemic stroke by making remote mechanical thrombectomies a reality. Visit website.

Matt Curran
CEO
-

NeoCor develops the CoApt Valve®, a transcatheter device designed to treat severe mitral and tricuspid valve disease through a minimally invasive approach. The CoApt Valve features a self-expanding nitinol frame and an electrospun polymeric valve, aiming to reduce or eliminate regurgitant jets without the need for open-heart surgery. The device is delivered via a catheter inserted through the transfemoral vein, preserving the native valve structure. CoApt Valve is advancing its technology through preclinical studies in both canine and human models, with plans to initiate veterinary clinical trials. Visit website.

George Kramer
Founder & CEO
-

NeoPredics develops advanced predictive clinical decision support solutions using machine learning and AI algorithms to assess maternal and neonatal health risks. Their primary focus is on early detection of conditions such as preeclampsia and jaundice, providing data-driven insights to improve outcomes in high-risk pregnancies and newborn care. Visit website.

Thorsten Waloschek
CEO
-

Neuroelectrics unlocks the power of computational neuroscience to treat brain disorders. Pioneering non-invasive tech for personalized neuromodulation, aiming to restore brain health & improve lives. Visit website.

Ana Maiques
CEO & co-founder
-

New Phase is advancing cancer treatment through a proprietary nanotechnology platform designed to improve outcomes for patients with stage IV cancer. The company’s approach uses biocompatible, multicore superparamagnetic iron oxide nanoparticles delivered to tumor tissue and activated by an alternating magnetic field to induce localized hyperthermia. By heating cancer cells to 50°C, this method selectively destroys tumors while sparing healthy tissue, aiming to extend life and preserve quality of life. Visit website.

Irie Meltzer
VP Business Development
-

Newronika's AlphaDBS is a closed-loop deep brain neuromodulation system. The device interprets the bioelectrical neuronal activity (local field potentials, LFPs) in the brain areas where stimulation is delivered and accordingly adapts moment-by-moment the stimulation to follow patient’s clinical condition. The technology is being developed as a treatment for Parkinson's disease. Visit website.

Keith Carlton
Executive Chairman
-

Newrotex, based in Oxford, UK is a clinical-stage startup redefining nerve repair with the first viable silk-based device for large-gap injuries. Our flagship product, SilkAxonsTM, solves the massive unmet need for an effective, scalable, large-gap peripheral nerve repair device —an untapped, multi-billion-pound market. Visit website.

Alex Woods
Founder, CEO
-

NewUro, with Bay Area partner Stellartech Research Corporation, has developed Uzap: a single-session, transurethral, drug-free and implant-free RF therapy for overactive bladder (OAB). Following a $2M NIH Fast-Track award, the system is now first-in-human-ready. We have applied for a $3M NIH Phase IIB continuation grant and are seeking additional funding to address the therapy gap in the multibillion dollar OAB market. Visit website.

Itzhak Avneri, MD
CEO
-

NIMBLE is developing a first-in-class, non-invasive, non-ionizing system to monitor coronary stent health using microwave-based technology. Designed to detect occlusion, fracture, or migration, the NIMBLE System provides cardiologists with objective data on stent status—without relying on invasive angiograms. With over 4 million stents implanted annually and a 30% failure rate within two years, NIMBLE addresses an $8B market with the goal of improving outcomes and saving lives. Visit website.
-small.jpg)
Oriol Iborra
CEO & Co-Founder
-

Nitinotes develops the EndoZip™, the first fully automated endoscopic suturing system for Endoscopic Sleeve Gastroplasty (ESG), a minimally invasive weight-loss procedure. Designed for efficiency, EndoZip enables a single-operator ESG procedure with automated, standardized suturing, reducing variability, procedure time, and training requirements. The system facilitates outpatient treatment with low-risk and predictable outcomes, addressing the growing need for effective, non-surgical obesity interventions. Visit website.

Lloyd Diamond
CEO
-

Njord is a medtech spin-off from Sahlgrenska University Hospital with a mission to solve unmet needs in radiology and patient handling. The first product launched is a novel patient transfer device (co-bot solution) Atle® 180 enabling improved efficiency and quality of care for acute care departments. The solution is already in use by +30 hospitals in Northern Europe. Visit website.
-small.jpg)
Jacob Ahrnstein
CEO & Co-founder
-

Noah Labs Vox eliminates the no. 1 cause of hospital admissions - congestive heart failure - 2 weeks before hospitalization by analyzing voice changes caused by fluid build-up in the lungs and vocal tract. Trained on the world's largest heart failure voice data set, the patented technology is being piloted at Mayo Clinic, UCSF and Charité Berlin. Visit website.

Oliver Piepenstock
CEO & Co-Founder
-
-small.jpg)
Nordstar Medical has developed a patented single-handed deployment device: a novel peripheral nerve block catheter (PNBC) designed for insertion before or after surgery to deliver local anaesthetic directly to targeted nerves. Rooted in nearly two decades of clinical research, this technology represents the first opioid-free, high-usability solution for managing postsurgical pain. Addressing a $56.5 billion global postoperative pain market, Nordstar is focused on U.S. and European orthopedic and anesthesia departments to improve recovery and reduce hospital length of stay (LOS) costs. Visit website.

Tore Allerup, MD
Co-founder and CEO
-

Severe infections still claim a huge number of lives, to date 11 million per year. This is partially caused by the limitations of current diagnostic methods, which are often slow and fail to identify the causative pathogens. As a consequence, clinicians have to rely on untargeted, broad-spectrum therapy which can be inadequate or associated with negative side-effects for the patient. Noscendo addresses this critical need with DISQVER® which leverages next-generation sequencing (NGS) of cell-free DNA, isolated from blood samples, to precisely identify 16.000 microbes and parasites. Visit website.

Andreas Kapplein
CEO
-

Nostics is developing point-of-care diagnostics that combine Raman spectroscopy, nanotechnology, and artificial intelligence to enable rapid pathogen identification. By detecting the molecular fingerprint of microorganisms, Nostics provides fast, affordable testing to support appropriate antimicrobial use and improve health outcomes. The platform’s AI-based classifier can be quickly adapted to recognize new pathogens, offering scalable solutions for global diagnostic needs. Visit website.

Eva Rennen
Co-Founder & COO
-

Novuson’s patented DTU technology will represent the first substantive innovation in over 25 years for targeting vessel sealing / dividing, and hemostasis (bleeding control) in surgical and trauma applications. Visit website.

Stuart Mitchell
CEO
-

Nuri is the first emotion brain-computer interface (eBCI) built for psychiatry, with initial focus on severe, treatment-resistant PTSD. Our implantable, therapeutic Deep Brain Recoding (DBR) system detects, decodes and retrains dysfunctional emotion circuits according to each patient's neural signature - delivering relief where conventional treatments fail. Visit website.

Dan Rappaport
CEO
-

Nyxoah is a medical technology company developing innovative neuromodulation therapies for obstructive sleep apnea (OSA). Its lead product, Genio, is a battery-free, minimally invasive hypoglossal nerve stimulation device implanted through a single incision and controlled by a wearable. Designed as an alternative to CPAP therapy, Genio has demonstrated best-in-class outcomes for reducing OSA burden. Nyxoah aims to transform sleep health for the over one billion people affected by OSA worldwide. Visit website.

Olivier Taelman
CEO
-

ORTHOSON’S Bio-Structural technology: injectable, anatomy-sparing cellular scaffold, combined with immediate mechanical restoration. Visit website.

Rich Simmonds
CEO
-

Osteal Therapeutics is a musculoskeletal therapeutics company developing solutions for the treatment of orthopedic infections. The company's lead product, VT-X7, is a drug-device combination that utilizes high concentrations of vancomycin and tobramycin, in conjunction with a drug delivery system, to irrigate infected joint spaces as a result of periprosthetic joint infection in joint replacement patients. Visit website.

David Thompson
CEO
-

Panda Surgical, a University College London spinout, is introducing the world's smallest robotics and AI platform for minimally invasive neurosurgery, offering surgeons enhanced dexterity and decision-support to make these operations safer, more effective, and more widely adopted. Visit website.

Manios Dimitrakakis
CEO
-

PanTher Therapeutics is a clinical-stage oncology company developing its Sagittari platform, a localized, polymer-based drug delivery system that enables sustained high-dose cancer therapy directly at tumor sites while minimizing systemic side effects. Visit website.

Laura Indolfi
CEO
-

Paradromics is building the world’s most capable and clinically viable brain-computer interface (BCI) platform. Its advanced neurotechnology captures brain activity at the highest resolution, individual neurons, enabling AI-powered treatments for motor impairment, chronic pain, addiction, depression, and other neurological conditions. The company's first clinical product, Connexus® BCI, is designed to restore speech and communication for people who are unable to speak due to debilitating motor conditions. Visit website.

Matt Angle
Founder and CEO
-

Peerbridge Health is a digital health company advancing cardiac care through AI-driven ambulatory ECG monitoring. Its flagship device, the Peerbridge Cor, is a patented 3-lead, 2-channel wireless AECG patch designed on Einthoven’s Triangle to replicate 12-lead Holter quality. The device captures 31 rhythms with exceptional signal fidelity, enabling rapid and remote heart failure diagnosis in as little as 30 minutes. By combining proprietary algorithms with high-resolution ECG recordings, Peerbridge Health offers a more accessible and efficient alternative to in-hospital screening for patients with chronic cardiovascular conditions. Visit website.

Chris Darland
CEO
-

Perfuze develops catheter-based technology for the treatment of acute ischemic stroke. The Millipede System enables efficient clot removal via an extra-large lumen aspiration catheter designed to maximize first pass success. The company recently completed its U.S. pivotal IDE study and has raised €54 million to date. Visit website.

Wayne Allen
CEO
-

Needle-free jet injection technology, proven for vaccine and therapeutic delivery, with appropriate clinical claims and regulatory approvals for intradermal and intramuscular tissues, now expanding into subcutaneous chronic disease indications with new platforms. Visit website.

Heather Potters
EVP, Co-Founder
-

PharmaSens is developing the world’s first patch for automated insulin therapy — integrating insulin infusion and continuous glucose monitoring in one device. Our solution will radically simplify diabetes management and cut costs by more than 50% compared to current options. Visit website.

Marcel Both
CEO
-

Pixee Medical develops augmented reality solutions for implant placement that offer orthopedic surgeons cutting-edge and clinically proven tools for precise and efficient surgery. Its first generation Kneeᐩ technology has already been used in over 10,000 procedures in more than 20 countries, demonstrating its positive impact on surgical practices worldwide. Visit website.

Sebastien Henry
CEO
-

Planatome is a patented technology platform, adapted from semiconductor manufacturing and licensed to industry leaders to create the world’s most advanced surgical cutting instruments – think semiconductor meets medical. Visit website.
-small.jpg)
Tim Tobin
CEO
-

Plexāā is a UK-based MedTech company developing innovative solutions to improve surgical outcomes. Its flagship device, BLOOM⁴³, is a fully wearable system designed to precondition the skin before breast surgery by gently warming the area to stimulate the release of Heat Shock Proteins, which improve blood flow and support healing. Clinical trials have shown that this approach can reduce wound healing complications such as infection and skin necrosis. Plexāā is also investigating applications of this technology in other surgical areas including obstetrics, orthopedics, and vascular surgery. Visit website.

Gaele Lalahy
COO
-

Polarean’s Xenon MRI platform, now commercially available in the United States for patients six years and older, uses the first and only FDA-approved inhaled contrast agent (XENOVIEW®) to provide functional lung imaging that visualizes regional ventilation and gas exchange without radiation. The platform integrates with MRI systems from GE HealthCare, Philips, and Siemens Healthineers, supporting earlier diagnosis, treatment planning, and monitoring of lung diseases. It also serves as a sensitive tool for drug development, enabling quantitative assessment of therapeutic response. Visit website.

Christopher von Jako, PhD
CEO
-

Precision Cardiovascular is focused on enhancing the outcomes and quality of life for patients with heart failure. The company is developing a miniature sensor that is placed in the pulmonary artery to continuously collect and analyze data to improve the remote management of cardiovascular diseases. Visit website.

Mohamed Abou-Alam
CEO
-

EASEE is Precisis' neurostimulation device that can act in two modes - excitatory and inhibitory. The duality of the device allows its to be used for the treatment of many cerebral diseases, including epilepsy, depression, and cognitive disfunctions caused by stroke. Precisis is currently developing a close-loop system for their EASEE technology, which will allow the device to record brain activity and respond with targeted stimulation. Visit website.

Karl Stoklosa
CEO
-

PBM-HALE™ is a non-invasive, contamination-free deep lung sample collection device. It enables clinicians and researchers to obtain distal lung fluid for laboratory analysis without procedures such as bronchoscopy or sputum induction. The samples can be used in clinical and research settings to investigate disease mechanisms, validate new diagnostics, and monitor treatment response, creating pathways to better patient outcomes across a wide range of respiratory conditions. Visit website.

Sterghios Moschos
Founder/Chief Executive Officer
-

Quantanosis.ai is developing a portable, AI-powered stroke detection and treatment platform that is non-invasive, operator-independent, and capable of enabling diagnosis and intervention in under two minutes. Visit website.

Yousef Khalili
Co-founder
-
-small.png)
QuantiLight develops an In-vitro Diagnostic platform for Therapeutic Drug Monitoring from home. Fueled by bioluminescent sensor technology from the Max-Planck-Institute for Medical Research, we provide actionable blood test results in just 15 minutes. Our secure app empowers chronic patients and healthcare professionals, reducing hospital visits and environmental impact, while saving costs to the healthcare system. Visit website.

Corentin Gondrand
CEO
-

RebrAIn is a medical technology company focused on improving Deep Brain Stimulation (DBS) and brain lesion surgeries for Parkinson’s disease and essential tremor. Its platform uses supervised AI algorithms and a collaborative health data registry to accurately identify brain regions targeted for treatment. By standardizing and streamlining pre-surgical planning, RebrAIn integrates AI-guided targeting into neurosurgical workflows to support greater precision and consistency in patient care. Visit website.

David Caumartin
CEO
-

Refined Laser Systems is a biophotonics company developing stain-free, molecular-specific microscopy technology that delivers H&E-equivalent tissue diagnostics within minutes to support faster, more reliable cancer surgery and treatment decisions. Visit website.

Max Brinkmann
Business Development
-

ReGelTec, Inc. is a clinical stage medical device company developing the next generation of interventional spinal implants for chronic lower back pain due to degenerative disc disease. Hydrafil is a Injectable hydrogel for Degenerative Disc Disease the procedure has a very short recovery time 1-2 hrs post injection with early clinical results showing significant improvement in back pain and ODI scores. Visit website.

Bill Niland
CEO
-

Rekovar develops wearable biosensor technology aimed at improving the management of Neonatal Abstinence Syndrome (NAS). Its flagship product, NeoMonki, is an investigational neonatal monitoring kit designed to provide real-time, objective data on infants' physiological and behavioral states. The system includes a wristband sensor, imaging camera, tablet application, and a provider portal, enabling healthcare professionals to deliver personalized care and intervention. Visit website.

Shiva Sharareh
CEO
-

Relief Srl is an innovative Italian MedTech company developing a first-in-class device for the treatment of stress urinary incontinence (SUI). Visit website.

Gioia Lucarini
CEO and Co-Founder
-
Resolve Stroke’s vision is to transform neurodiagnosis from a complex, delayed process into one that is rapid and reliable at the point of care. Its software suite converts raw ultrasound data into neurovascular biomarkers, enabling earlier and more accurate diagnosis in time-critical settings. This biomarker platform defines a new diagnostic category and creates scalable licensing opportunities with OEMs and healthcare providers. Visit website.

Aritz Zamacola
cofounder and CEO
-

RespirAI is developing a wearable platform that passively monitors physiological data to support long-term patient care. Originating from research at the University of Nebraska Omaha, the system uses a novel bio-coupling marker to measure synchronization between breathing and walking—enabling early detection of COPD exacerbations. Its algorithm integrates artificial intelligence to enhance predictive accuracy, helping patients, families, and clinicians anticipate changes in respiratory health. Visit website.

Nimrod Bin-Nun
Co-founder & CEO
-

Retia develops healthtech AI algorithms for helping guide management of surgical and critically ill patients to improve outcomes. This is a $4 billion market. The company's technology has been clinically validated and is used in 65 hospitals. Visit website.

Marc Zemel
CEO
-

ReVision Implant is developing the world's first prosthesis that restores useful vision to blind people. We are using implants with thousands of microelectrodes and advanced stimulation algorithms to directly transmit video to the visual cortex of the brain. Visit website.

Frederik Ceyssens
co-founder & CEO
-

Rob Surgical has developed Bitrack, a portable surgical robotic system designed for use across multiple operating rooms and hospitals. Its compatibility with standard ORs and pay-per-use model eliminate the need for dedicated infrastructure or high procedural volumes, enabling broader access to robotic surgery in underserved markets. Visit website.

Mario Ferradosa
CEO & General Manager
-

SamanTree Medical's Histolog Scanner is a digital microscopy scanner for high-resolution imaging of the surface of fresh tissue. The device utilizes novel, ultra-fast confocal microscopy technology to enable rapid histological assessment in the operating room or pathology laboratory. Visit website.

Olivier Delporte
CEO
-

Scalpel AI builds a connected surgical intelligence platform with AI-driven systems for the surgical supply chain and sterile services, focusing on instrument recognition, tray validation, and workflow automation. Their technology helps hospitals, orthopedic and spine device companies, distributors, and sterile processing divisions to reduce errors, streamline logistics, and improve instrument readiness. Visit website.

Dr. Yeshwanth Pulijala
Founder & CEO
-

ScienceForBrain is developing a medical technology designed to protect brain function during cardiac bypass surgeries and neuro-intensive care. Built on over 30 years of research and multiple patents, the system enables heart-lung machines to generate regular rectangular blood flow pulsations, allowing real-time assessment of cerebral autoregulation. This capability helps clinicians maintain optimal brain perfusion tailored to each patient, with the aim of reducing postoperative complications such as delirium and cognitive decline. Visit website.

Tad Svendrys
Chief Operating Officer
-

Seerlinq is the first certified medical device to offer non-invasive, cost-effective monitoring of cardiac filling pressure from home. The telemonitoring service can detect heart failure decompensation up to 30 days in advance, helping to prevent hospitalizations and deaths using just a simple oximeter or smartwatch. Seerlinq has been shown to reduce hospitalizations by up to 80%, making it one of the most effective non-invasive interventions. Already reimbursed and in clinical use across Slovakia, Seerlinq is now entering the German market and partnering with a leading smartwatch brand to scale heart failure diagnostics and monitoring globally. Visit website.

Allan Bohm
CEO
-

Transforming the experience for both patient and clinician in interventional procedures with a 3D augmented reality platform featuring real-time holographic visualization of the patient’s actual anatomy, “floating” over the patient. Visit website.
-small.jpg)
Berk Tas
CEO
-

Sequana Medical NV develops technologies to treat diuretic-resistant fluid overload in liver disease, heart failure, and cancer. Its alfapump device, approved by the FDA as a breakthrough and PMA-approved, is a fully implantable pump that automatically moves excess fluid to the bladder for natural excretion, targeting recurrent ascites from liver cirrhosis. Sequana Medical is also advancing DSR, a phase IIa drug program aimed at treating diuretic resistance and cardiorenal syndrome in heart failure. Both technologies address large and growing markets driven by increasing disease prevalence. Visit website.

Ian Crosbie
CEO
-

Serox is developing low-cost, non-invasive IVDs for high-incidence diseases at the point of care. By combining Raman Spectroscopy, Surface Enhanced Raman Spectroscopy (SERS), and machine learning, Serox enables the detection of cancers and infections in urine without the need for invasive procedures like blood draws or cystoscopy. The company is initially focused on bladder, prostate, and kidney cancers, with plans to expand into women’s cancers and urinary tract infection diagnostics. Serox’s technology aims to streamline screening and reduce unnecessary interventions through fast, accurate, and personalized urine-based testing. Visit website.

Mark Evans
CFO
-

Shape Memory Medical is focused on innovative, therapeutic solutions for endovascular markets with its proprietary shape memory polymers (SMP). SMP is a bioabsorbable polyurethane embolic scaffold that supports rapid conversion to organized thrombus followed by tissue ingrowth. With commercialized devices in peripheral vascular embolization space, the company is actively leveraging its platform technology to develop novel applications for the management of complex aortic pathologies including aortic aneurysms. Visit website.

Edward “Ted” Ruppel
President and CEO
-

SitnStand is a mobility technology company focused on enhancing independence for individuals with limited strength or mobility. The company’s flagship product is a personal lift assistant that helps users safely and confidently transition between sitting and standing. Designed for use at home or on the go, SitnStand offers a practical solution for maintaining autonomy without relying on caregivers. Visit website.

Gal Goldner
CEO
-

SmartCardia, a Swiss med-tech company has developed a next-generation 7-lead ECG and vitals patch and cloud platform for real-time cardiac and remote patient monitoring. With FDA and CE approvals, proprietary AI for arrhythmia detection, and a U.S. diagnostic facility enabling reimbursement, the company is positioned for rapid global growth. Visit website.

Srinivasan Murali
CEO
-

Solaris Endovascular, Inc. is a growth stage, US-based medical device company developing advanced endovascular solutions for patients suffering from dialysis access challenges and Peripheral Artery Disease. With a proven stent platform and innovative drug-eluting technology, led by seasoned executives with extensive experience in cardiovascular MedTech, Solaris Endovascular is uniquely positioned and committed to improving long-term patient outcomes and transforming vascular health. Visit website.

Randy Hubbell
President & CEO
-

Solenic Medical is developing a non-invasive medical device that utilizes alternating magnetic fields to eradicate biofilms that form on implants. The company's platform uses external magnetic coils to destroy infective biofilms on the surface of knee and hip replacement implants, as well as plates and rods for trauma related procedures. Solenic's solution is being developed to replace the expensive and risky two-step revision surgery, which is the current standard of care for chronic infections on medical implants. Visit website.

Bart Bandy
CEO
-

SonicPrecision is developing a dual-source RF and PFA ablation catheter with integrated ultrasound sensors that enable real-time monitoring and visualization for safer, more precise treatment of cardiac arrhythmias. Visit website.

Rene Aarnink
CEO
-

Sonion Health is developing a biosensing digital health platform for cuffless blood pressure monitoring, addressing hypertension as a major risk factor for cardiovascular mortality. The technology has been developed over several years by Sonion engineers, known for their expertise in balanced armature receivers, premium microphones, and electromechanical devices. This platform aims to enhance hypertension management through continuous, non-invasive monitoring.

Jim Fidacaro
Executive Vice President, Sonion & CEO
-

The first osseointegrative biomimetic knee cartilage implant. Visit website.

Dimitrios Angelis
Co-Founder
-
-small.png)
SpinaFX is bridging the therapeutic gap in the treatment of back and leg pain caused by a contained herniated disc. The company has pioneered a minimally invasive, image-guided treatment that injects a mixture of oxygen and ozone gas to reduce a single level herniation. Clinical data shows that the solution leads to a reduction of disc volume, which relieves nerve root compression to alleviate pain. Visit website.

Jeff Cambra
CEO
-

Spinally is developing an intrathecal spinal cord stimulation and recording system that significantly improves on existing neuromodulation solutions. Unlike conventional products, Spinally’s technology offers superior spatial precision and deeper stimulation capabilities while consuming less power. It also enables high-fidelity neuronal signal recording, supporting closed-loop approach. The device is designed for rapid deployment using a micro-needle insertion method, which simplifies the surgical process and minimizes patient recovery time. Our initial focus is chronic pain. However, the system's design and performance open opportunities across a broader range of therapeutic applications: Parkinson’s disease, neurorehabilitation, and spine-computer interface, creating a path toward treating complex neurological conditions with greater specificity and adaptability. Visit website.

Pawel Soluch
CEO
-

StarTric’s mission is to advance and transform the treatment of tricuspid regurgitation, a serious and significantly undertreated heart valve disease. Building on the scientific and clinical foundation of a proven surgical technique, the company is developing TriClover—a transcatheter device designed to deliver a safe, effective, and minimally invasive solution for a broad range of patients, including those ineligible for open-heart surgery or current transcatheter therapies. Visit website.

Eugenio Passanante
Co-founder & CFO
-

STENTiT is developing a novel class of regenerative implants, solely composed from resorbable fibers. The balloon expandable implant provides instant structural support to keep the artery open, while allowing circulating blood cells to infiltrate into the porous mesh. This triggers a natural healing response by which immune cells gradually replace the mesh with new vascular tissue as the implant safely dissolves over time. Hereby, the RFS fully reconstructs the artery from the inside out. In addition, the fibrillated design allows to be scaled beyond 100 mm to serve the majority patient population. Visit website.

Bart Sanders
CEO & Founder
-

Surfix is developing a photonic diagnostics platform that combines a nanocoated biochip, microfluidic cartridge, and desktop reader to deliver rapid, high-precision point-of-care protein detection with expansion potential for molecular testing. Visit website.

Jos Lunenberg
CEO
-

SurgiBox Inc. develops technology to improve surgical safety for both patients and healthcare providers, particularly in resource-limited settings. The company’s flagship product, SurgiBox, is designed to reduce intraoperative contamination of incisions while minimizing provider exposure to bodily fluids and aerosols. SurgiBox redefines surgical safety by focusing protection on the surgical field rather than the entire operating room. The company continues to develop a pipeline of products aimed at enabling safe and efficient surgical care in diverse environments. Visit website.

Mike Teodorescu
CEO
-

Synseer is a wearables company developing biometric devices designed to help users better manage their personal health and wellness. Its flagship product, HealthBuds, are in-ear devices that simultaneously track hearing and heart health to deliver continuous, high-accuracy vital sign data. Supported by an AI-powered wellness assistant, HealthBuds translate body signals into actionable insights, helping users take a proactive approach to their health—simply by wearing earbuds. Visit website.

John Martino
Founder, CEO
-

Tempo Therapeutics is a San Diego-based biotechnology company advancing tissue engineering through its proprietary MAP (Microporous Annealed Particle) material science technology. The platform enables the in vivo formation of functional tissue and organs and supports the development of next-generation vaccines that are shelf-stable and multivalent. MAP scaffolds are fully synthetic, minimally invasive, and designed to evade immune rejection while promoting a pro-regenerative immune response. Tempo’s mission is to use MAP to help define the future of medicine. Visit website.

Westbrook Weaver
CEO
-

Tensive is a biomedical startup, which develops innovative scaffolds aiming to offer a natural breast reconstruction or augmentation. Tensive’s long-term mission is to restore a natural breast to patients, who experienced reconstructive surgery after mastectomy or lumpectomy, to improve their quality of life and avoiding recurrent and expensive surgical operations. Visit website.

Sanjay Kakkar
CEO
-

The BioSport™ is an AI-powered, multi-omics platform that merges genetics, biomarkers, and wearable data to optimize athletic performance and safety. Its unique BioFit™ Score, analyzing 15,000+ sport-related variants, delivers hyper-personalized training, nutrition, and recovery plans. Athletes and coaches gain data-driven real-time insights via The BioSport interactive App to enhance results, reduce injury risk, and unlock peak potential. Visit website.

Raman Kapoor
Chief Healthspan Officer and BioGut
-

The Insides Company is a first-in-class medtech company providing breakthrough therapeutic devices for intestinal failure patients. Visit website.

Garth Sutherland
CEO
-

ThinkSono Guidance enables any non-ultrasound trained healthcare professional to scan for blood clots (DVT). It reduces waiting times, negative DVT cases and can be used at the point of care. Visit website.

Fouad Noor
CEO
-

Titanium Textiles AG is the first company worldwide producing elastic titanium for medical applications, usually implants. Based on a broad medical device registration in a major country there are many studies available and thousands of patients were treated with excellent results. The production technology is proprietary and the company has a significant number of issued application patents. The technology will substitute many products so far based on Polypropylene. Visit website.

Christoph Ehlers
President & Founder
-

TFO - Tricuspid Flow Optimizer A Novel Transcatheter System for Minimally Invasive Treatment of Tricuspid Regurgitation Visit website.

Stéphane Piat
Chairman of the Board
-

TYBR Health is developing a natural hydrogel to prevent adhesions (internal scarring) that can occur following minimally invasive surgery. The technology is intended to reduce post-operative complications which can cause patients pain and require rehabilitation following tendon and joint surgeries. Visit website.
-small.jpg)
Tim Keane
Co-Founder & CEO
-

Cultivating brain health therapy through continuous, ultra-long-term EEG monitoring and transformative real-world data. Our MDR CE-approved 24/7 EEG SubQ solution captures subcutaneous brain activity to deliver actionable insights that empower clinicians and researchers to personalize treatment and reduce uncontrolled seizures in epilepsy. By harnessing AI and big data, we are unlocking new possibilities in digital biomarkers, drug development, and neuromodulation. Visit website.

Martin Stenfeldt
CEO
-

VACERE Medical’s feasibility trial in vasospasm subjects reduced the rate of death and disability from a U.S. national average of 50% — to 0%. Equally impressive: CT imaging in each study subject confirmed “on-command” (i) enhanced perfusion and (ii) vasodilation. VACERE intends to pursue accelerated clearance in vasospasm and commence revenue, while gathering data to demonstrate prophylactic (and non-invasive!) elimination of cerebral vasospasm as a disease state. FDA granted an IDE for the emergency treatment of ischemic stroke patients, which VACERE will also pursue. Please talk with us about joining our fight against cerebral vasospasm and stroke! See the two-minute video at vacere.com. Visit website.

Jeffrey Lietzke
CEO
-

Vascular Perfusion Solutions specializes in the development of technologies to enhance the preservation of organs. The VP.S ENCORE device is designed to extend the viability of organs for transplantation beyond the current standard of care. The device consists of a single-use disposable unit that operates with a non-blood, nutrient-rich solution. VPS is currently focused on developing the device for use in heart transplantation, with plans to modify the device for the preservation of other organs, ex vivo therapies, and organ banking. Visit website.

Rafael J. Veraza
CEO & President
-

Vena Vitals is an early stage med-tech company focused on non-invasive continuous blood pressure monitoring. The company's proprietary technology is a soft flexible sensor that conforms to the skin for direct pressure measurement at theradial artery. The company's technology is intended to replace unnecessarily invasive arterial line measurements as well as non-continuous cuff-based methods in both the hospital and at home. Visit website.

Ray Liu
CEO & Co-Founder
-

With the rapid growth of large-bore transcatheter procedures such as TMVR, TTVR, and leadless pacemaker implantations, there is a critical unmet need for safe, effective, and time-saving vascular closure solutions. Venock addresses this gap with a proprietary vascular closure device capable of closing access sites as large as the vessel diameter itself (20-30F and larger). Venock device features a novel, staple-based mechanism that is quick and easy to deploy, and without any complicated preparation steps. Visit website.

Wolfgang Goetz
Founder
-

Our novel pVAD technology is the most powerful and smallest pVAD technology that we know, able to provide over 7L/min at physiological pressures and provide total left ventricular support for cardiogenic shock and other critically ill patients. Total support has been shown to reduce infarct size for heart attack patients, and the recent DanGer shock trial has validated the use of pVAD technology in cardiogenic shock patients. The technology is based on 7 granted patents and no catheter or sheath larger than 9Fr is used in the percutaneous, Cath lab, implant procedure. Visit website.

Martin Cook
CEO and co-founder
-

Versono is developing an intravascular ultrasonic platform to improve the crossing of severe occlusions in the peripheral vasculature. The device will prepare a working lumen to support endovascular therapy in patients with chronic total occlusions (CTOs) and critical limb ischemia (CLI). Visit website.

Finbar Dolan
CEO
-
-small.jpg)
The VESTECK, "SUTURE-TIGHT"tm endovascular catheter is a platform technology that comes preloaded with nitinol sutures. Our initial target market is improving the durability of implants in the $7B aortic repair space. Improving implant durability for patients, providing better results for clinicians and reduced costs for the global health care system. Visit website.

Joseph Rafferty
CEO and Founder
-

ViCentra is a commercial-stage company redefining wearable insulin delivery with Kaleido, the smallest, lightest, and most accurate insulin patch pump in its class. Designed to empower rather than remind users of their chronic condition, Kaleido combines consumer-grade aesthetics with clinical precision—discreet, customizable, and built for real life. Integrated with Diabeloop’s CE-marked closed loop algorithm, Kaleido enables fully automated insulin therapy. ViCentra is actively scaling manufacturing to meet surging demand, with more than 3,200 users across Germany, France, and the Netherlands and a goal of reaching over 12,000 users by the end of 2026. The company is headquartered in Utrecht, The Netherlands, and is preparing for U.S. market entry in 2027. Visit website.

Tom Arnold
CEO
-

VirtaMed develops mixed-reality surgical simulators designed to enhance medical education and improve patient outcomes. Its platforms integrate real surgical instruments with haptic feedback and photorealistic graphics to provide immersive training experiences across specialties such as orthopedics, obstetrics and gynecology, urology, and general surgery. VirtaMed also offers customizable training solutions for medical device companies and educational institutions, supported by its cloud-based platform, VirtaMed Connect, for performance tracking and curriculum development. The company is headquartered in Zurich, Switzerland, with additional locations in the United States and China. Visit website.

Raimundo Sierra
CEO & Co-Founder
-

A spin-out from the University of Oxford, Vivid Dx is a precision diagnostics company transforming pathogen detection and antimicrobial resistance (AMR). The company is developing a next-generation platform to deliver broad pathogen ID in 30 minutes and phenotypic antibiotic susceptibility testing (AST) in 3 hours – directly from blood, with no blood culture required. By combining novel pathogen cell isolation, Raman spectroscopy, a proprietary Raman spectra database, and advanced AI/ML, the platform has the potential to redefine the speed, accuracy, and clinical utility of infectious disease diagnostics. The company is backed by Oxford Science Enterprises and other leading investors. Visit website.

John Sperzel
CEO
-

Wellumio is a venture-backed company specializing in portable MRI solutions for acute neuroimaging applications. The company’s core enabling technology is a novel pulse-gradient free nuclear magnetic resonance sensor platform with an initial focus on acute stroke triage and diagnosis. Visit website.

Dr Shieak Tzeng
Co-founder & CEO
-

Wellvii is a digital health company focused on improving patient care through innovative remote monitoring solutions. Its flagship product, the Wellvii BP Go, is a cuffless, portable blood pressure monitor that makes tracking heart health simple, comfortable, and accurate. By seamlessly connecting patients and providers through real-time data, BP Go helps improve compliance, early detection, and long-term health outcomes. Visit website.

Mark Khachaturian
CEO
-

WISE S.p.A. is developing next-generation electrodes for neuromonitoring, neuromodulation, and brain-machine interfaces using its proprietary Supersonic Technology. The company’s portfolio includes the FDA-cleared WISE Cortical Strip for intraoperative monitoring and the Heron® lead, the first percutaneous directional lead for spinal cord stimulation. WISE’s thin, flexible electrodes offer superior neural tissue conformity and minimal invasiveness. These innovations aim to disrupt the $3B SCS market and advance care across multiple neuro applications. Visit website.

Luca Ravagnan
CEO
-

Xeltis' bioabsorbable devices, made of bioabsorbable polymers that are structured as porous matrices through electrospinning, are designed to work as valves or vessels once implanted, and to allow ETR as the body's natural healing process pervades them with components of native tissue, including collagen, endothelial lining and capillary blood vessels, which normally develop and organize themselves into natural functioning tissue. The matrices are structured to be absorbed overtime, to leave patients with new, healthy, functioning valves or vessels. Visit website.

Eliane Schutte
CEO
-

Yaqrit is developing an extracorporeal liver support system aimed at critically ill patients with multiple organ failures. The system removes blood toxins and replaces dysfunctional albumin to support recovery from acute-on-chronic liver failure (ACLF). Clinical data indicates a 67% improvement in patient outcomes over 10 days. Visit website.

Troels Jordansen
CEO
-

YourHeartCheck provides Direct to Consumer ECG Holter analysis and develops technology that combines continuous ECG monitoring with algorithms capable of estimating blood pressure every ten heartbeats. Over a standard 72-hour Holter recording, this produces approximately 24,000 blood pressure data points, providing a detailed picture of cardiovascular behaviour alongside rhythm analysis. Visit website.

Chris Crockford
Founder
-

Zedsen’s mission is to provide safe, affordable, reliable breast health screening for all women. Our low cost, non-radiating, compact hardware, combined with AI-enabled analysis, makes this possible. Zedsen’s technology not only provides cancer screening, but can also assess breast density, allowing women to avoid inappropriate follow up screening. Non-radiating tech means women can get assessed as often as they like, greatly improving early detection rates, empowering women to safely and regularly monitor their breast health. Visit website.

Alexander Antrobus
CEO
-

Zepto Life Technology is developing a next-generation molecular diagnostics platform that leverages Nobel Prize-winning Giant Magnetoresistance (GMR) technology to deliver rapid, ultra-sensitive, and multiplexed detection of infectious diseases. The company aims to transform diagnostic standards by enabling high-precision, point-of-care testing with faster turnaround times than traditional methods. In addition to its core platform, Zepto operates diagnostic labs and provides CRO services to support FDA submissions and commercialization for other diagnostic developers. Visit website.

Hannah Zhang
CEO
-

ZygoFix develops and commercializes its zLOCK system, a proprietary technology that adds "bend-ability" to joints and provide natural stabilization. The technology is currently used to achieve spinal fusion and spinal stability without screws. Visit website.

Ofer Levy
CEO
voices to the conversation







































































-(51).png)
-(28).png)
---2025-07-01T175343.652.png)
-(1).png)
.png)
-(3).png)
-(4).png)
-(56).png)
-(6).png)
-(55).png)
-(8).png)
-(9).png)
-(23).png)
-(54).png)
-(12).png)
-(53).png)
-(14).png)
-(32).png)
-(33).png)
-(17).png)
-(25).png)
-(35).png)
-(34).png)
-(21).png)
-(41).png)
-(42).png)
-(43).png)
---2025-07-01T092918.275.png)
-(37).png)
-(25).png)
-(26).png)
-(34).png)
-(27).png)
-(36).png)
-(29).png)
-(30).png)
---2025-07-01T180110.546.png)
-(38).png)
-(39).png)
-(40).png)
---2025-07-01T180355.781.png)
-(36).png)
-(37).png)
---2025-07-01T181020.773.png)
-(39).png)
-(31).png)
-(41).png)
-(42).png)
-(49).png)
-(44).png)
-(46).png)
-(50).png)
-(46).png)
-(48).png)
-(45).png)
-(44).png)
-(48).png)
-(52).png)
.png)
-(1).png)
-(47).png)

-(3).png)
.png)
-(30).png)
-(5).png)
-(6).png)
-(7).png)
-(8).png)
-(9).png)
-(10).png)
-(11).png)
-(12).png)

-(13).png)

-(14).png)
-(15).png)
-(16).png)
-(18).png)
-(19).png)
-(20).png)

.png)


-small.jpg)
-small.jpg)
-small.jpg)









-small.jpg)
-small.jpg)


-small.jpg)
-small.jpg)

-small.jpg)



-small.jpg)





-small.jpg)
-small.jpg)

-small.jpg)
-small.jpg)





-small.jpg)

-small.jpg)


-small.jpg)







-small.jpg)


-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)




-small.jpg)
-small.jpg)






-small.jpg)







-small.jpg)


-small.jpg)

-small.jpg)



-small.jpg)




-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)

-small.jpg)
-small.jpg)





-small.jpg)
-small.jpg)
-small.jpg)


-small.jpg)

-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)

-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)

-small.jpg)
-small.jpg)


-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)

-small.jpg)
-small.jpg)
-small.jpg)




-small.jpg)









-small.jpg)




-small.jpg)




















-small.jpg)
-small.jpg)
-small.jpg)



















-Appelbaum---Novian-Health-usa-small.jpg)





-small.jpg)


-small.jpg)
















































































































































-small.jpg)









-small.jpg)





























































































































_(48)-small.jpg)



































-small.jpg)


-small.jpg)



-small.jpg)










_(26)-small.jpg)


























-small.jpg)



-small.jpg)

-small.jpg)

-small.jpg)
-small.jpg)

_(48)-small.jpg)


-small.jpg)











-small.jpg)

-small.jpg)
-small.jpg)












-small.jpg)







-small.jpg)





-small.jpg)






-small.jpg)

-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)


-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)

-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)








-small.jpg)






-small.jpg)



-small.jpg)
-small.jpg)

-small.jpg)

-small.jpg)
-small.jpg)

-small.jpg)
-small.jpg)


-small.jpg)
-small.jpg)
-small.jpg)




-small.jpg)




-small.jpg)








_(67)-small.jpg)





-small.jpg)




-small.jpg)











-small.jpg)


-small.jpg)
-small.jpg)
-small.jpg)

-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)
-small.jpg)













-small.jpg)
-small.jpg)

-small.jpg)
-small.jpg)




-small.jpg)




