
Under the direction of CEO Martin Stenfeldt, UNEEG Medical is building a remote patient monitoring platform that captures longitudinal EEG in real-world settings and transforms it into actionable insight for epilepsy care and research. Based in Copenhagen, the company’s EpiSight solution pairs an implanted sensor with an end-to-end data workflow to help clinicians understand seizure patterns over time, accelerate review, and make more informed decisions that support therapy optimization for patients living with epilepsy.
Origin Story
UNEEG Medical began 20 years ago with a different clinical target. The company’s roots trace back to HypoSafe, founded in 2005 by Professor and Chief Physician Henning Beck-Nielsen and Rasmus Stig Beck Jensen. The original concept was designed to detect hypoglycemia in people with type 1 diabetes by monitoring brain activity and issuing early warnings before blood sugar levels became critical.
“Henning, a professor in diabetes, was inspired by the push to commercialize university research and began exploring whether continuous EEG could be used to identify hypoglycemia and provide instant alerts,” Stenfeldt explained.
As the technology matured, the team discovered the implant could deliver exceptionally high-quality signals over long periods. That insight led UNEEG to reassess where the technology could have the greatest clinical impact.
“When continuous glucose monitors became widespread, we realized the bigger unmet need was in epilepsy,” Stenfeldt said. “That pivot was strongly inspired by the Epilepsy Foundation, which awarded us the Shark Tank Prize in 2017. That was really the start of the journey toward what we do today.”
Stenfeldt, a serial entrepreneur and author, joined the company as CEO about a year ago after first working with UNEEG through an advisory role tied to fundraising and investor expansion.
The Current Landscape
UNEEG is focused on a persistent gap in epilepsy care: the lack of objective, real-world EEG that reflects how patients actually live.
“Today’s EEG tools are largely anchored to short clinical snapshots, ranging from routine one-hour recordings to expensive multi-day inpatient monitoring stays,” Stenfeldt explained. “In the hospital, patients often hope they will have a seizure so clinicians can observe it, but that is the opposite of how they live day to day.”
He emphasized that hospital environments can unintentionally distort the data clinicians rely on. “Monitoring is often done under artificial conditions where seizures may even be induced. What clinicians really need is a longitudinal view of patients in their normal lives. That includes sleep, work, exercise, and everyday routines.”
This gap becomes especially important for the most complex cases. “Around 40% of people with epilepsy continue to suffer from uncontrollable seizures, despite trying at least two anti-seizure medications,” Stenfeldt said. “For those patients, physicians need deep insight into seizure patterns, what happens during and after events, and how sleep and daily life affect outcomes.”
Today, much of that insight depends on patient-reported seizure diaries. “The current gold standard is still paper-based diaries, which are notoriously underreported or overreported,” he said. “That makes therapy optimization slower and more difficult than it needs to be.”
Inside the Innovation
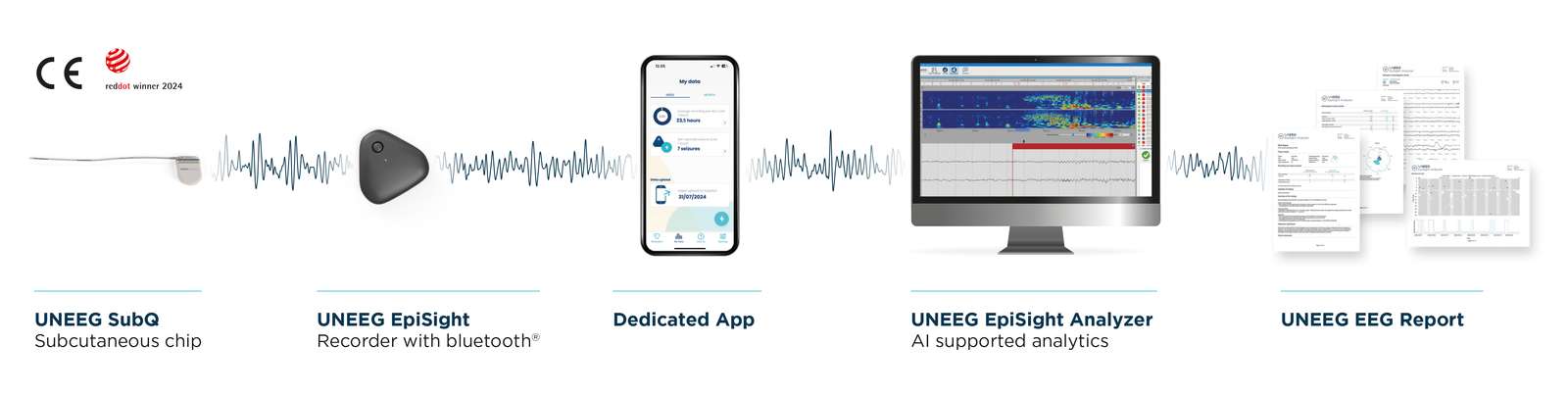
UNEEG’s EpiSight solution is designed as an end-to-end system that enables continuous EEG monitoring outside the clinic. The platform includes the SubQ implant, EpiSight Recorder, EpiSight App, and EpiSight Analyzer.
“The minimal invasive implant is placed just under the skin behind the ear and measures EEG signals through a set of electrodes,” Stenfeldt said. “It is a passive device designed to remain in place for years. In Europe, we are currently approved for three years.”
An external recorder powers the implant and automatically begins collecting data when attached. “The recorder acts as the battery and holds roughly 48 hours of charge,” he said. “From there, data flows to the patient’s smartphone and securely into our cloud platform.”
Continuous monitoring creates an enormous volume of information. UNEEG uses machine learning algorithms to filter noise and highlight clinically relevant events. “When you monitor EEG 24/7, you generate huge amounts of data,” Stenfeldt said. “Our algorithms help remove noise and identify suspected seizures and relevant activity.”
The goal is to transform how clinicians interact with long-term EEG data. “Traditionally, it can take about an hour to review a single day of EEG,” he said. “Our solution makes it possible to review a full month of EEG data in an hour, with clinicians validating and annotating what the system surfaces.”
For UNEEG, the implant is only one part of the broader vision. “The implant is an important enabler, but the real value is in the data and the insights it provides,” Stenfeldt said. “We are focused on helping physicians optimize treatment and helping researchers better understand brain health.”
Progress and Milestones
UNEEG has already achieved early commercialization in Europe. “We have CE MDR approval and early sales across several European countries,” Stenfeldt said. “Those early launches have given us valuable learning about customer needs and evidence requirements.”
The company has now submitted its FDA application and is preparing for U.S. commercialization. “The U.S. is a major priority for us,” he said. “Our experience in Europe has helped us refine our approach and better prepare for entering a larger market.”
One of UNEEG’s most significant milestones is the scale of its real-world EEG dataset. “We have accumulated more than 450,000 hours of real-world EEG, including 10,000 annotated seizures and 150,000 hours of sleep data,” Stenfeldt said. “It is an unprecedented dataset, and it continues to grow.”
That data is already driving new opportunities. “We are using it to improve seizure characterization and develop future capabilities such as seizure forecasting and real-time alerts,” he said. “It also creates opportunities to collaborate with drug developers and neuromodulation companies that need objective longitudinal data.”
UNEEG is also expanding its clinical footprint. “An investigator-sponsored study in Italy has begun exploring pediatric use, including the first child implanted with our device,” Stenfeldt said.
Looking ahead, UNEEG is preparing for its next phase of growth. “We are raising $50 million to support U.S. commercialization,” Stenfeldt said. “Our goal is to continue building the real-world EEG foundation that can drive better therapy decisions and expand possibilities across brain health.”
Join Us at LSI USA ‘26
Stenfeldt has been selected to present at LSI USA ‘26, March 16th–20th, in front of hundreds of global medical technology companies. Join us in welcoming him to the event in Dana Point, CA, where he will share the latest updates on UNEEG’s technology and development.

Schedule an exploratory call
Request Info17011 Beach Blvd, Suite 500 Huntington Beach, CA 92647
714-847-3540© 2026 Life Science Intelligence, Inc., All Rights Reserved. | Privacy Policy










