In a successful week for the medical technology companies in the LSI Alumni community, we saw LSI Alumni appoint new executives, achieve regulatory milestones, and much more on the road to LSI Europe ‘25 in London (September 7-11).
Axoft
Announced a strategic partnership with Kayaku Advanced Materials to co-develop specialty polymers for biomedical engineering and bioelectronics. The collaboration expands Axoft’s materials platform and supports its mission to translate lab-developed innovations into clinical impact.
Biobeat
Appointed LSI Alumni Raymond W. Cohen, former CEO of Axonics, as the new chair of the board of directors. Cohen brings decades of leadership experience across multiple medtech companies, supporting Biobeat’s next phase of growth in ambulatory patient monitoring.
Centerline Biomedical
Appointed Jim Dillon as President and CEO to lead the next phase of growth for the IOPS navigation and visualization platform. Dillon brings extensive cardiovascular medtech leadership experience, succeeding Gulam Khan, who oversaw the development and clearance of Centerline’s second-generation devices.
CMR Surgical
Appointed Chris O’Hara as Commercial President & General Manager – US to lead the company’s commercial strategy following FDA marketing authorization of the Versius surgical robot. O’Hara brings two decades of experience in the medical device industry, including senior roles at Virtual Incision, Intuitive Surgical, and Globus Medical.
Egg Medical
Published new study results in JSCAI demonstrating that its EggNest Complete radiation shielding system provides superior protection for the entire interventional team. The findings highlight Egg Medical’s commitment to improving occupational safety by significantly reducing radiation exposure for all staff, not just the operator and assistant, during X-ray guided procedures.
Nalu Medical
Released its next-generation Therapy Disc, a 39% smaller wearable power source for its Nalu micro-IPG. Nalu says that the launch enhances patient experience and creates an option for more patients seeking relief from chronic pain with peripheral nerve stimulation therapy.
Onward Medical
Received an FDA Investigational Device Exemption (IDE) to initiate the Empower BP pivotal trial of its ARC-IM system for spinal cord injury patients. The study will assess the implantable neuromodulation platform’s ability to stabilize blood pressure, targeting individuals with chronic orthostatic hypotension and autonomic dysreflexia.
Pleural Dynamics
Completed enrollment in a post-market clinical study evaluating its automatic effusion shunt (ACES) system, the first fully implantable ACES that uses motion from normal breathing to remove fluid from the chest. The study supports U.S. market release plans and highlights the potential of ACES to improve the quality of life for patients.
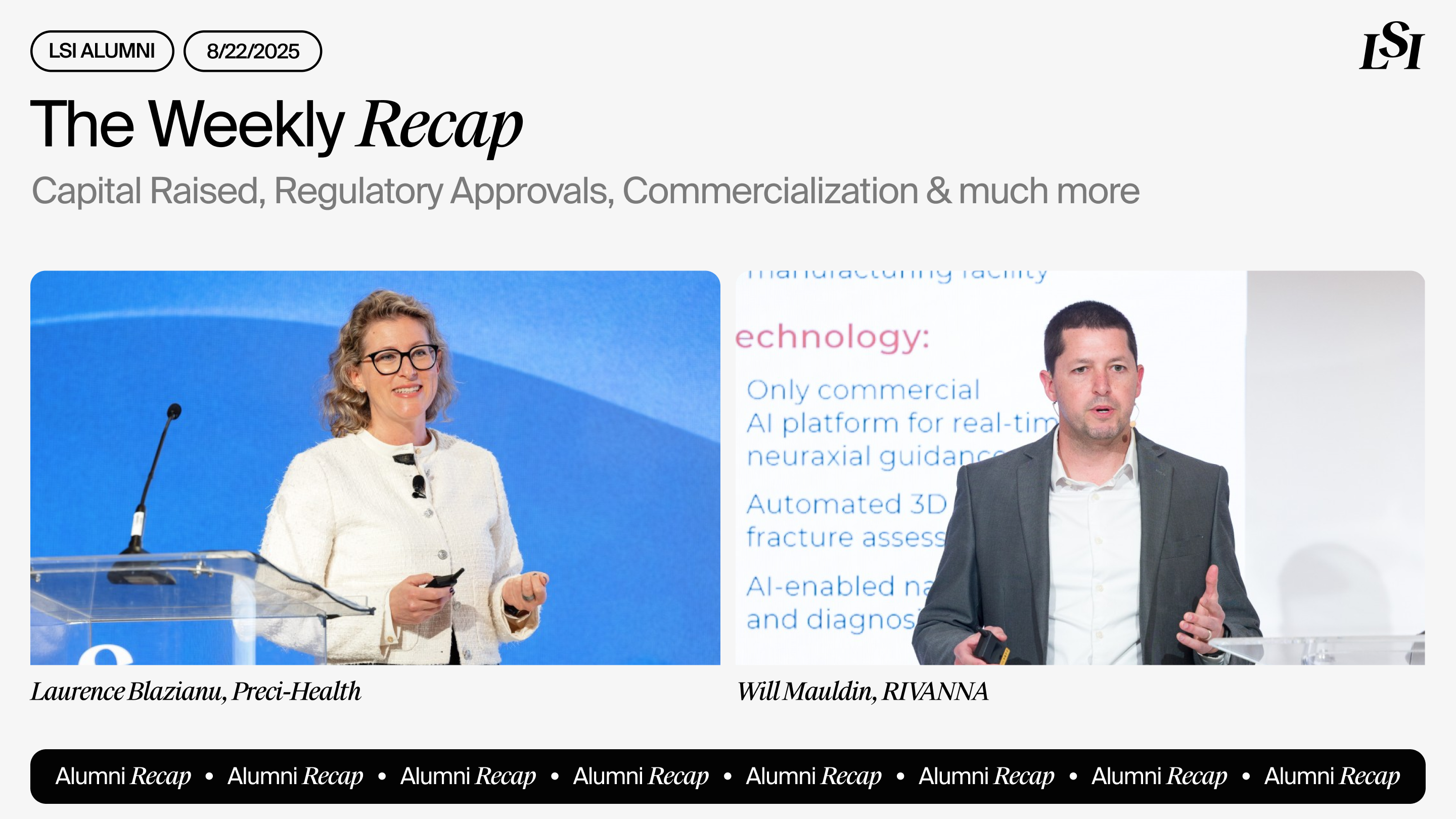
Preci-Health
Appointed Paul Kirchgraber, MD, MBA, as Chairman of the Board to help advance strategic and commercial objectives. The former Labcorp Drug Development CEO brings decades of leadership experience in clinical trials and diagnostics to support Preci-Health’s mission of transforming blood collection with its needle-free EZdraw technology.
RIVANNA
Was awarded an $800,000 Virginia Catalyst Grant, with $1.6 million in matching funds, to advance its AI-enabled Accuro 3S ultrasound system for neuraxial anesthesia. The funding supports the finalization and clinical validation of advanced product features.
SpinaFX
Received FDA Breakthrough Device designation for Triojection, a minimally invasive, image-guided procedure that utilizes a proprietary oxygen-ozone delivery system for treating contained lumbar disc herniations. The designation recognizes Triojection’s potential to improve safety, access, and outcomes globally, validating over 20 years of research and development.
Synchron
Appointed Andy Rasdal as Chief of Staff and Mark Brister as VP of R&D to support U.S. clinical trials and global commercialization of its Stentrode BCI. The executive additions bring decades of medical device leadership and product development expertise to Synchron as it advances its BCI for people with severe paralysis.
Stay tuned for more updates, insights, and achievements of our LSI Alumni in The Weekly Recap, and follow our blog for the latest medical device news and advancements.

Schedule an exploratory call
Request Info17011 Beach Blvd, Suite 500 Huntington Beach, CA 92647
714-847-3540© 2026 Life Science Intelligence, Inc., All Rights Reserved. | Privacy Policy