
Under the direction of CEO and Co-Founder, Dr. John Santini, Vergent Bioscience is advancing a molecularly targeted intraoperative imaging agent designed to help surgeons see tumors in real time during minimally invasive and robotic-assisted procedures. With its lead product, abenacianine for injection (VGT-309), the clinical-stage company is focused first on lung cancer, where improved tumor visualization can meaningfully impact surgical outcomes. As Vergent prepares to enroll its Phase III clinical trial, advancing the product toward regulatory approval, the company is positioning its technology for expansion across additional solid tumors and global oncology markets.
Origin Story
“Vergent didn’t have a single founder, but a founding team,” Santini said. Early collaborators Jeff Julkowski, an angel investor, and Dean Banks, an early-stage venture creator and investor, came together through longtime medtech entrepreneur Doug Kohrs. Their initial focus was on pre-clinical biological imaging applications outside of regulated human use.
Santini was recruited early on to define and execute the company’s strategy. “The scientist in me was fascinated by the precision enabled by molecular targeting, and my inner entrepreneur was excited about all the business opportunities that the technology could create,” he said. Within months, however, the company made a pivotal shift. “It became clear to me that the real opportunity was not in pre-clinical markets. It was in surgical oncology.”
That pivot reshaped Vergent’s mission. “We decided to take this right turn and focus our efforts to help surgeons do a better job finding tumors in real time during surgery,” Santini said. What began as a novel protease-targeting mechanism invented by Professor Matt Bogyo of Stanford University has since progressed to late-stage clinical development. “Vergent coupled a small, experienced team with a molecule on a whiteboard that produced promising early data, and now we’re heading toward a Phase III study in lung cancer,” he said.
The Current Landscape
Minimally invasive and robotic-assisted surgeries continue to expand across surgical oncology because they reduce tissue trauma, blood loss, and recovery time. At the same time, these approaches introduce new challenges for surgeons.
“Minimally invasive and robotic procedures take the surgeon’s hands out of the patient, decreasing their ability to see and feel tissue,” Santini said. “Removing the surgeon’s direct contact with tissue puts them at a disadvantage in finding early-stage or hidden disease.” This challenge has become more pronounced in lung cancer, where surgeons are increasingly moving from removing entire lobes of the lung to tissue-sparing sublobar resections.
This shift benefits patients, but it makes tumor localization and margin identification more difficult. “If you don’t know exactly where the tumor is, it becomes much harder to do these tissue-sparing procedures with confidence,” Santini said.
“We chose lung cancer [as our initial indication] because of the vast unmet need in this disease, the importance of surgery to the treatment of lung cancer, and the opportunity to make an immediate and meaningful impact for these patients,” he said. While overall lung cancer incidence is declining, Santini noted an increase in early-stage diagnoses, where surgery can be curative if tumors are fully resected.
From a market perspective, Santini pointed to an estimated 75,000 lung cancer surgeries annually in the U.S., representing an initial opportunity of roughly $240 million. Globally, this figure grows to approximately 430,000 surgeries and nearly a $2 billion lung cancer opportunity. When the use of abenacianine is expanded beyond lung cancer to the other solid tumor cancers it targets, the global market opportunity increases to a staggering $20 billion.
Inside the Innovation
Vergent’s lead product, abenacianine for injection, is a tumor-targeted, intraoperative imaging agent designed to provide real-time surgical guidance. “Our goal is to help surgeons visualize tumors and make sure they get them all out during surgery,” Santini said. “If you achieve a complete resection, surgery for lung cancer can be curative.”
Unlike conventional tumor localization methods, Vergent’s approach interrogates tumor biology directly. The agent targets cathepsins, a family of proteases that are highly overexpressed in a wide range of solid tumors, enabling broad applicability beyond lung cancer. “We chose this molecule because it gives us the ability to target not just one type of tumor, but all solid tumors,” Santini said, pointing to future opportunities in breast, colorectal, prostate, and other cancers.
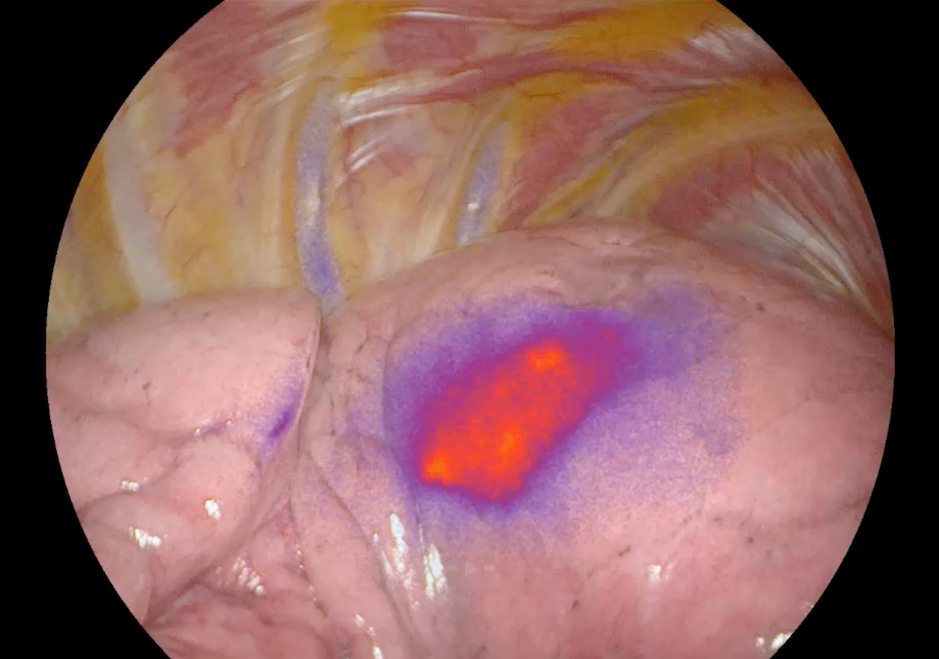
A key differentiator is that abenacianine is activatable. “When this agent is administered to the patient, it stays dark. It only lights up when it binds to its enzyme target at the tumor,” Santini explained. This activation mechanism minimizes background signal and creates contrast between the tumor and surrounding healthy tissue. The imaging component incorporates ICG, or indocyanine green, making the agent compatible with commercially available near-infrared imaging systems already in use in operating rooms.
“In our clinical trials, we don’t measure success by how often abenacianine lights up tumors, as this happens nearly all the time. Instead, success is measured by how often we provide surgeons with actionable information in real-time that can help with their decision-making during the procedure,” he explained. In the Phase IIb multicenter VISUALIZE study, 45% of patients had at least one clinically significant event, or CSE, defined as the occurrence of any one of the following: (i) localization of tumors not found by standard surgical techniques, (ii) discovery of previously unknown additional cancers confirmed by pathology, (iii) identification of inadequate surgical margins, or (iv) detection of cancerous lymph nodes. In other words, in 45% of surgical cases, the use of abenacianine provided real-time, actionable information to surgeons that they could use to improve the surgical procedure.
Santini emphasized that this type of insight addresses a fundamental limitation in surgery. “People assume surgeons can easily see cancer, but many tumors are small, below the surface, and visually indistinguishable from normal tissue,” he said. “When you turn on near-infrared imaging and see the differentiation abenacianine provides, it changes everything.”
Looking ahead, Santini envisions Vergent’s technology as part of a broader evolution in the operating room. “The future OR is going to combine mechanical systems like robotics with molecular information and AI,” he said. “We generate a critical biological data stream, and these data will ultimately feed into AI algorithms, enabling smarter surgical decision-making.”
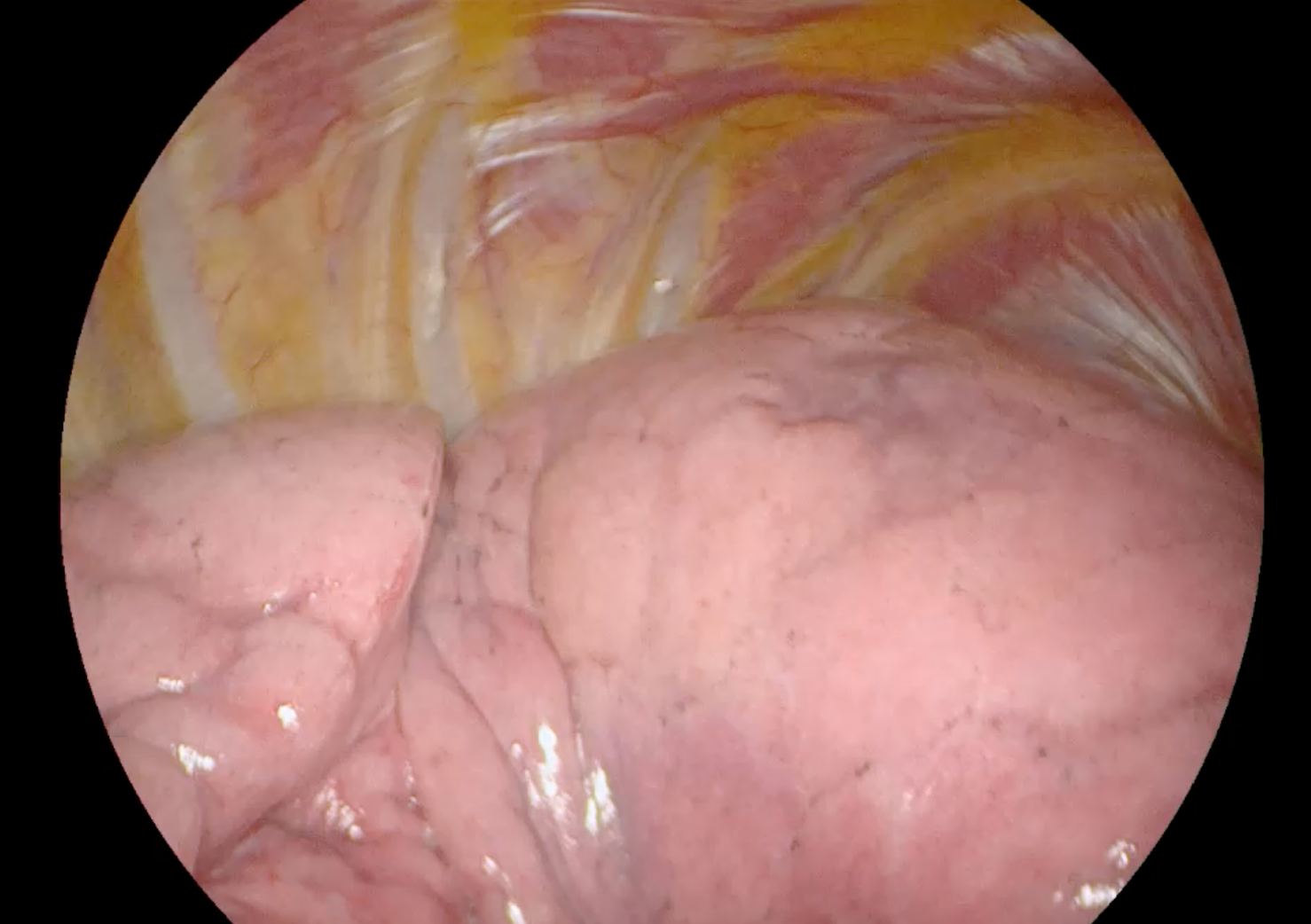
Lung visualization with standard white light
Lung tumor localization using abenacianine with NIR
Progress and Milestones
Vergent is entering its Phase III clinical trial, the last remaining trial before regulatory submission. “We expect to begin enrolling patients toward the end of the first quarter of 2026 and complete the study by the end of 2026,” Santini said.
The company is also raising a Series C round to support U.S. regulatory approval, commercial launch, and early expansion into additional tumor types and geographies. Santini highlighted Vergent’s capital efficiency as a defining characteristic of its development approach to this point. With a small but experienced team of seven full-time employees, Vergent has advanced its surgical oncology platform from concept to Phase III at a cost of approximately $40 million. “We’ve been intentional about remaining focused, operating efficiently, and doing a lot with very little, giving us the flexibility to direct more of our capital toward building shareholder value through commercial and corporate development activities,” he said.
Beyond lung cancer approval, future milestones include generating early clinical results in other solid tumor indications and expanding clinical and commercial efforts internationally.
The LSI Effect
For Santini, LSI stands apart from other industry gatherings. “LSI has quickly become the gold standard,” he said, contrasting it with larger conferences where meaningful interaction can be difficult. “What Scott and the team have created is an environment that brings the right people together in a format that is conducive to real conversations and encourages the building of lasting business relationships.”
Join Us at LSI USA ‘26
Santini has been selected to present at LSI USA ‘26, March 16th–20th, in front of hundreds of global medical technology companies. Join us in welcoming him to the event in Dana Point, CA, where he will share the latest updates on Vergent Bioscience’s technology and development.

Schedule an exploratory call
Request Info17011 Beach Blvd, Suite 500 Huntington Beach, CA 92647
714-847-3540© 2026 Life Science Intelligence, Inc., All Rights Reserved. | Privacy Policy










