
Under the direction of Founder and Managing Director Dr. Subhasis Banerji, SynPhNe is bringing a groundbreaking paradigm shift to neurological rehabilitation. With its FDA-cleared, wearable neuro-integrative platform, the company is enabling stroke and brain injury survivors to restore function years beyond the standard recovery window. “SynPhNe digitally restores independent living to those affected by stroke or brain injury,” said Banerji. “It trains brain and muscle in one integrated wearable system, bringing enhanced outcomes, access, and affordability to the doorsteps of those who need it the most.”
Origin Story
The invention of SynPhNe began with Banerji’s own recovery from a life-altering brain injury. “I beat all odds to make a full recovery after the medical community had given up,” he said. “In the course of this recovery, I realized there is a powerful brain plasticity route to recovery, a paradigm that could change how therapy has been traditionally done for the past 150 years.”
Inspired by the early success of clinical trials using a rudimentary prototype, Banerji launched the company as a spin-off from Nanyang Technological University, Singapore, with his PhD supervisor, Dr. John Heng. Later joined by Tarun Mathur, the two now co-lead SynPhNe’s mission to transform outcomes for patients around the world.
“As a caregiver for my grandparent who was a neuro patient and having worked under a boss who had a stroke, the subject was close to my heart,” said Mathur. “I watched a session with SynPhNe at a local hospital that had all the cutting-edge technologies and realized SynPhNe had a unique potential, something I had not seen or anticipated before.”
The Current Landscape
The scale of neurological disease is staggering and growing. “Stroke is the number one reason for healthcare-related bankruptcy in the U.S.,” said Banerji. “There are about 700,000 new patients each year added to an existing population of seven million in the U.S. alone. The global burden is close to 100 million patients living with various grades of disability post-stroke.”
Despite this, the standard of care remains outdated and limited. “In most parts of the world, including many parts of the U.S., the standard of care is still manual therapy,” said Banerji. “It’s manpower-dependent, and the ratio of therapists to patients significantly skews the ability to deliver an adequate dosage of therapy, even in the best of healthcare systems.”
Available technologies such as robotics and neuromodulators are expensive, complex to operate, and generally not suitable for home use. SynPhNe, by contrast, is designed for accessibility and affordability, making effective therapy possible both in the clinic and at home.
Inside the Innovation
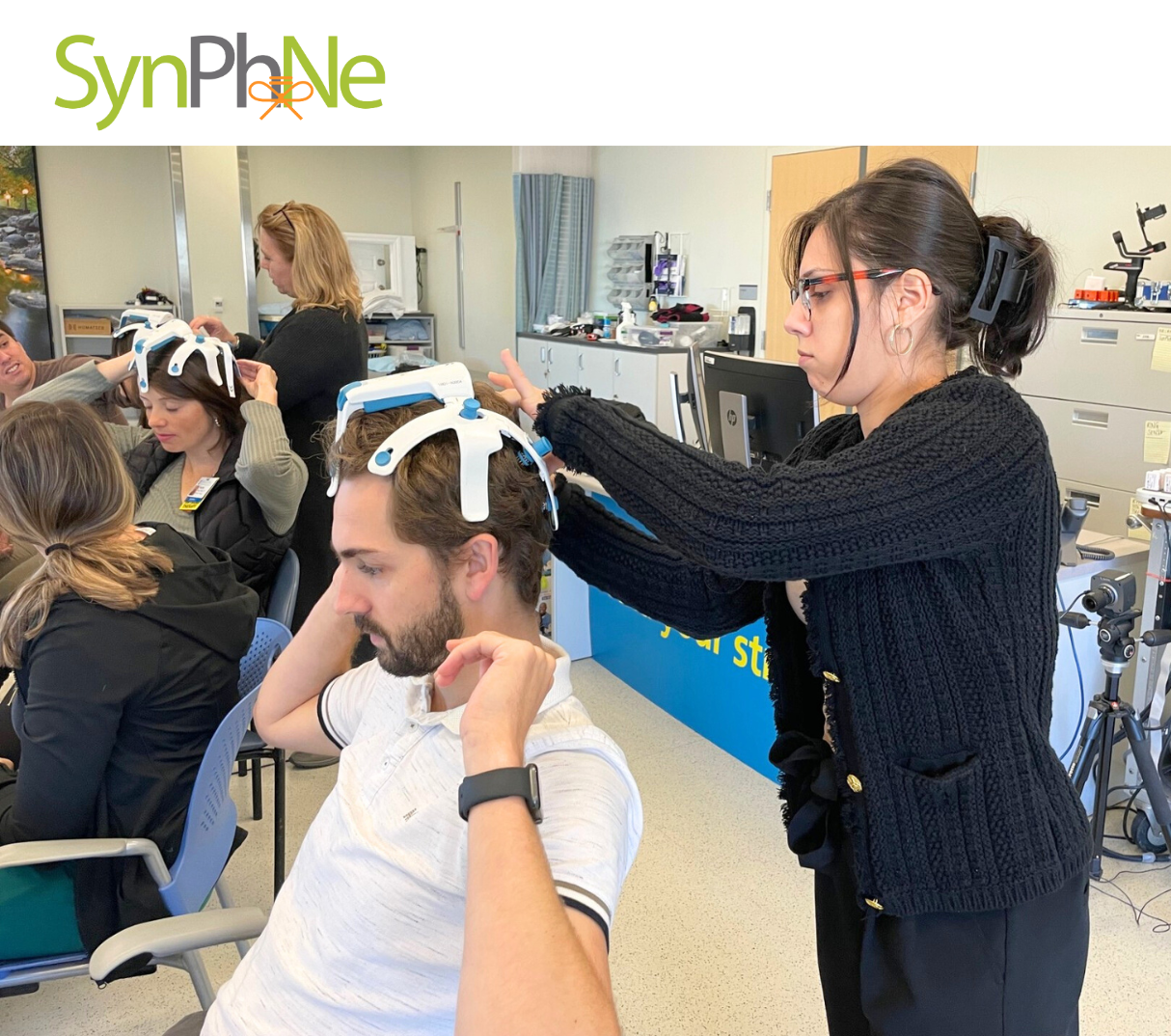
At its core, SynPhNe’s wearable platform captures time-synchronized EEG and EMG signals through soft, dry sensors embedded in a headgear and armgear system. These real-time brain and muscle signals feed into an intuitive software interface that runs a patented “Feedforward-Feedback” learning model.
“The software enables learning in an exploratory and goal-directed manner,” Banerji explained. “It synchronizes how the brain and muscles work together, rather than in conflict. Training modules help break maladaptive compensation traps and rebuild functional task performance like reading, writing, eating, dressing, and typing.”
The results appear quickly. “The first micro-changes start to appear as early as two to three sessions,” he said. “In early stages of stroke, we’ve seen significant improvement in independence measures within eight to 15 days before discharge, as compared to standard care.”
Unlike traditional approaches, SynPhNe shows measurable impact even in late-stage chronic patients. “While most of the world believes that a recovery window closes after 12 months, SynPhNe has restored function up to 10 years post-stroke,” said Banerji.
“Our proprietary metrics shift patients from maladaptive compensation to a precise recovery pathway,” he added. “It is the world’s first and only neuro-integrative wearable that trains both brain and muscle in one system.”
Progress and Milestones
SynPhNe was officially listed on the FDA website in 2018–2019 and received its first hospital adoption in Singapore. In 2019, it won the World Innovation Cup in Medical Applications at Medica and was the only finalist from Asia, among 15 finalists.
After completing early clinical validation, the company launched its commercially manufactured device in the U.S. in January 2025, now available at three active provider sites.
Recent momentum includes raising the first part of their Series A and filing patents in new domains such as mental health and emotional dysregulation. The company also plans to commercialize adjacent applications in sports peak performance and learning disability, both massive global markets in their own right.
“We are raising the second part of our Series A, focused on scaling in the U.S.,” Banerji said. “This round will get us to our first $2 million in revenue in stroke and brain injury domains, and position us to launch two adjacent applications.”
Join Us at LSI USA ‘26
Dr. Banerji has been selected to present at LSI USA ‘26 next March 16th–20th in front of hundreds of global medical technology companies. Join us in welcoming him to the event in Dana Point, CA, where he will share the latest updates on SynPhNe’s technology and development.

Schedule an exploratory call
Request Info17011 Beach Blvd, Suite 500 Huntington Beach, CA 92647
714-847-3540© 2026 Life Science Intelligence, Inc., All Rights Reserved. | Privacy Policy










