Under the direction of Scientific Advisor Liesl Nel-Themaat, PhD, Noor Sciences is advancing a new standard for embryo and egg assessment in IVF through a fully non-invasive, AI-driven platform designed to deliver objective, actionable insight at the most critical decision points in the fertility journey. With deep clinical IVF experience and academic rigor, Nel-Themaat supports human-stage studies and identifies strategic opportunities as the company works to improve success rates, reduce patient burden, and unlock new diagnostic markets in reproductive medicine.
Origin Story
Nel-Themaat joined Noor Sciences as a scientific advisor following her role as the Clinical Associate Professor, Obstetrics & Gynecology - Reproductive Endocrinology & Infertility at Stanford University, where initial validation studies for the technology were conducted. What captured her attention was a fundamental gap in reproductive medicine that has persisted despite rapid growth in fertility services. As she explained, “What excited me about joining Noor Sciences was the founding team’s insight into a critical gap in reproductive medicine: despite a 30x increase in egg freezing since 2012, there’s no reliable way to assess egg viability before fertilization, an $8 billion untapped market.”
She was equally compelled by Noor’s approach to embryo viability assessment. “Current methods are either subjective or invasive, contributing to low IVF success rates,” Nel-Themaat said. “Noor’s first product addresses this head-on with a comprehensive embryo assessment device that combines metabolic profiling with AI-enhanced morphological grading using novel, non-invasive microscopy. This gives clinicians objective data to select the most viable embryos, which could significantly improve IVF outcomes while reducing the emotional and financial toll of failed transfers."
The Current Landscape
IVF success rates have remained stubbornly flat for years. “IVF success rates have been stuck at around 30% across all ages for years. Current embryo assessment methods are part of the problem, in that patients are often required to do multiple transfer cycles before a viable pregnancy is established,” Nel-Themaat noted. “Today, clinics rely on subjective visual grading by embryologists or invasive, expensive genetic testing such as PGT-A, which can cost $5,000 to $7,000 per cycle and introduces additional risk to the embryo.”
The consequences are significant. “One in six women faces infertility, yet 95% of infertile couples avoid IVF altogether due to the high costs and discouraging success rates,” she emphasized. “We're solving this by providing clinicians with objective, non-invasive tools to assess both egg and embryo viability, which could dramatically improve outcomes while making treatment more accessible and less emotionally taxing for patients."
From a market perspective, Noor Sciences says it’s entering a $48 billion global infertility market, with an estimated $25 billion serviceable addressable market in IVF diagnostics. The company plans an initial U.S. launch beginning with California clinics following FDA clearance, before expanding nationally and internationally.
Inside the Innovation
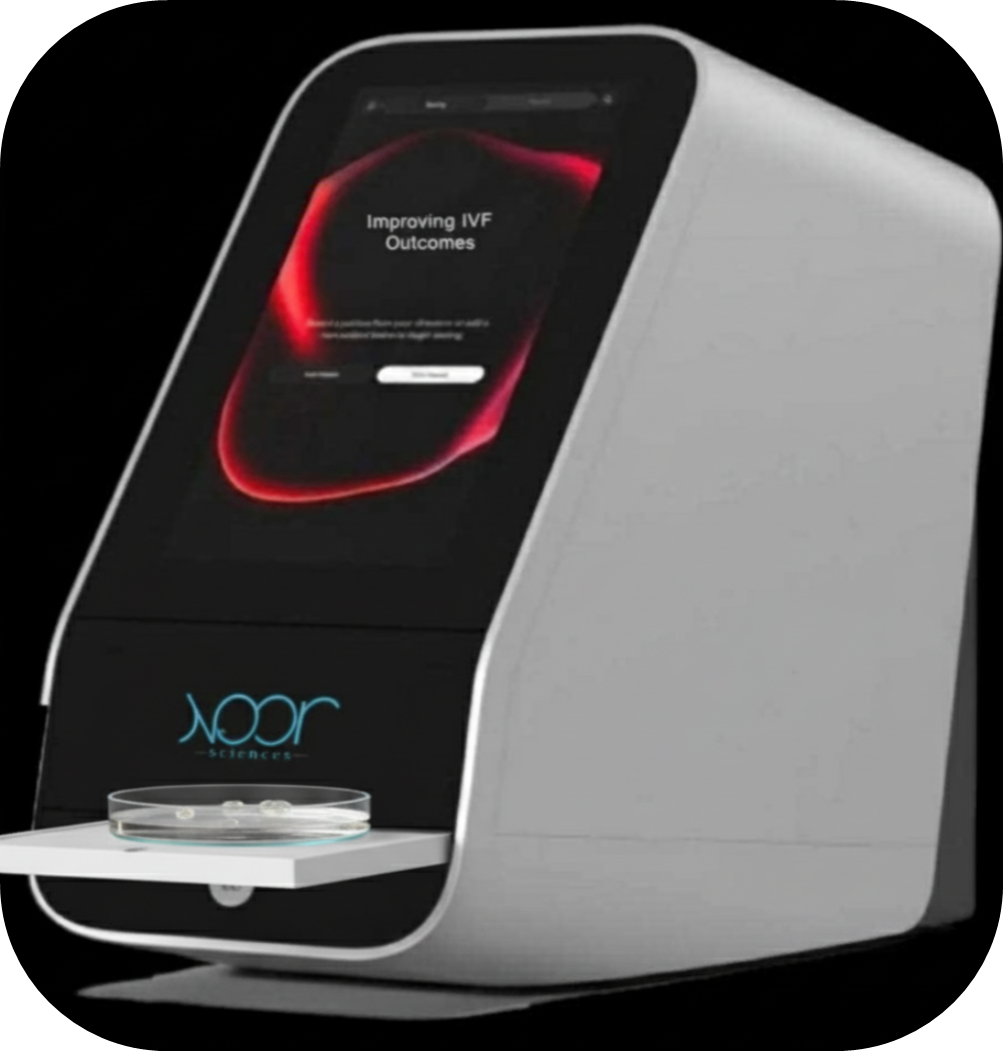
At the core of Noor’s platform is the EM-Lux™ system, powered by the proprietary EMMI, or Embryo Morpho-Metabolic Index, algorithm. “Noor’s non-invasive device, EM-Lux, combines a smart imaging dish with our patented AI platform to assess embryo viability in a completely new way,” Nel-Themaat explained.
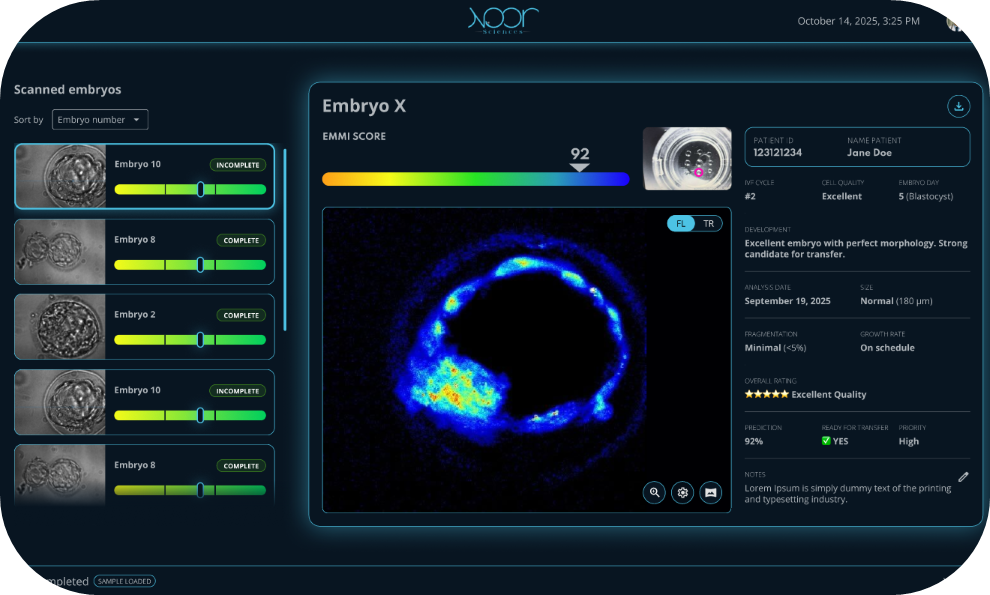
The system captures metabolic profiling in real time by analyzing enzymes and coenzymes that are directly tied to cellular energy production and metabolic health, then integrates that data with conventional morphological markers such as embryo shape, size, and developmental stage. “Our AI algorithm processes all of this information and generates a comprehensive viability score from 0 to 100, allowing clinicians to rank embryos by their likelihood of successful implantation,” she said.
What differentiates Noor is its focus on the embryo’s metabolic processes, not just how they appear. “We’re not just looking at what an embryo looks like; we’re assessing how it’s actually functioning at the metabolic level, which is a significant predictor of viability,” Nel-Themaat said. “It’s the difference between judging a car by its exterior versus checking under the hood to see if the engine is running properly.”
The system is designed to integrate seamlessly into standard IVF lab workflows. “It uses a custom optical system with dual-path imaging: one path captures brightfield images for morphological analysis, while the second path performs spectroscopic analysis to measure metabolic activity at specific, ultra-gentle wavelengths that don't harm the embryo,” she explained. Computer vision algorithms extract detailed morphological features, spectroscopy quantifies metabolic markers, and an ensemble AI model first segments viable versus non-viable embryos, then ranks viable embryos to guide transfer decisions. The platform operates with full automation and minimal supervision from embryologists, enabling high-throughput clinical use without disrupting existing protocols.
Progress and Milestones
Noor Sciences is in late-stage product development and following a design-control pathway toward FDA submission in Q1 2027. The company has completed human studies on approximately 300 embryos at Stanford-affiliated and other academic IVF centers, demonstrating both safety and efficacy. Its hardware prototype is fully functional and has been tested in real-world IVF lab settings.
From an intellectual property standpoint, Noor holds one granted patent with three additional patents pending. The team continues to collect data to refine and strengthen its AI models ahead of regulatory submission.
Commercially, Noor plans a controlled launch with five select IVF clinics following FDA clearance to validate its commercial model and generate real-world performance data before expanding to 20 California clinics in 2027 and 60 clinics nationwide by 2028. The company is currently raising a $7 million Seed round expected to close in Q2 2026, funding FDA clearance and initial commercial launch.
Looking ahead, Noor is building a broader fertility diagnostics platform. “Think of us as building the Illumina of fertility, but for live-cell metabolic imaging rather than genetic sequencing,” Nel-Themaat said. The roadmap includes embryo viability assessment as the foundation, egg assessment capabilities launching in 2028, and potential non-invasive alternatives to preimplantation genetic testing later in 2029. By 2030, the company aims to partner with more than 500 clinics globally and conduct over one million assessments annually, laying the groundwork for data-driven reproductive medicine at scale.
Join Us at LSI USA ‘26
Nel-Themaat has been selected to present at LSI USA ‘26, March 16th–20th, in front of hundreds of global medical technology companies. Join us in welcoming her to the event in Dana Point, CA, where she will share the latest updates on Noor Sciences’ technology and development.

Schedule an exploratory call
Request Info17011 Beach Blvd, Suite 500 Huntington Beach, CA 92647
714-847-3540© 2026 Life Science Intelligence, Inc., All Rights Reserved. | Privacy Policy