Armed with a brain-computer interface built from a novel ultra-soft material and bolstered by FDA Breakthrough Device Designation, Axoft is taking on neurological disorders, starting with disorders of consciousness as its first clinical indication. With first-in-human cases now complete, CEO and Co-Founder Paul Le Floch shares how Fleuron, their proprietary implant material, is unlocking clinical potential—and a new frontier in neurotechnology.
Reimagining the Interface Between Brain and Machine
Neurological disorders affect nearly one in three people worldwide. Yet, for many, there is no drug, therapy, or even a reliable diagnosis. Enter Axoft, a neurotechnology company led by Paul Le Floch, who is on a mission to change that. The Harvard University-trained materials scientist and Forbes 30 Under 30 honoree is reengineering how the brain and technology communicate—starting with a radically soft implant and a bold plan to decode the natural language of the brain.
“Our goal is to unlock the natural language of the brain by capturing the activity of single neurons,” said Le Floch. “To do that, we had to build a new class of brain-computer interface from the ground up.”
Inside the Innovation
That innovation is Fleuron—Axoft’s proprietary implant material that mimics the mechanics of brain tissue. It’s up to 10,000 times softer than polyimide—which is used in state-of-the-art BCIs—and 1,000,000 times softer than silicon. “Rigid implants damage and move in the brain,” Le Floch explained. “That causes scarring and makes it impossible to get stable, high-density data long-term.”
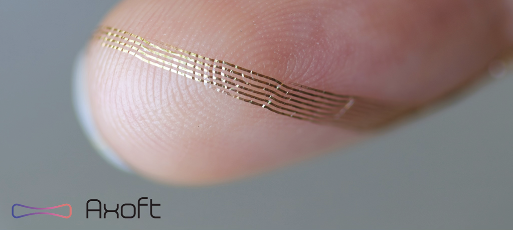
Fleuron changes that. Designed for seamless integration with neural tissue, the material minimizes glial encapsulation around the implant and moves with its natural pulsations—rather than resisting them. “They are so soft you can see how they bend around my fingerprints in this image,” said Le Floch. (See image on next page.)
“Rigid implants damage and move in the brain. That causes scarring and makes it impossible to get stable, high-density data long-term.”
By minimizing glial encapsulation and micromotion, Fleuron enables Axoft’s 1,024-sensor interface to maintain high-fidelity signal quality across time and brain regions previously considered inaccessible.
This scalable access to brain activity is central to Axoft’s long-term goal: training foundation AI models on rich neural datasets. “The brain is a data organ,” Le Floch said. “And now, we finally have a way to capture the information needed to decode it.”
First-in-Human Data
Earlier this year, Axoft completed its first-in-human clinical study at The Panama Clinic. The implants were tested during brain tumor surgeries, allowing researchers to evaluate the safety and neural recording in tissue already scheduled for removal.
“We planned for everything to go well—but it’s still a new technology,” said Le Floch. “What’s most exciting is that it all worked exactly as expected.”
The team demonstrated successful signal capture from patients under anesthesia and while awake, setting the stage for Axoft’s first indication: building a reliable prognostic for disorders of consciousness.
Tackling a Silent Epidemic
Disorders of consciousness, like vegetative or minimally conscious states, affect more than 500,000 patients each year in the U.S. and Europe. These patients are often caught in diagnostic limbo, with over 40% misdiagnosed due to reliance on behavioral exams that can miss signs of awareness. A recent study shows that more than 25% of patients may have cognitive-motor dissociation following a coma—conscious but unable to respond. “In these situations, the standard of care [to determine the condition of the patient and predict their recovery] is almost like flipping a coin to make life-altering decisions,” Le Floch said. “We want to bring more clarity and precision to that process.”
The need isn’t just clinical—it’s financial. Neuro-ICU stays cost $3,000 to $10,000 per day, and long-term care for patients in a vegetative state can exceed $100,000 per year. For those with severe traumatic brain injuries, lifetime care costs often surpass $2 million per patient.
“In these situations, the standard of care is almost like flipping a coin to make life-altering decisions.”
Axoft’s BCI platform offers a fundamentally new approach: a continuous bedside monitoring system capable of identifying brain states in real time—opening the door for more accurate diagnosis, prognosis, and communication.
The company’s first indication represents a $15 billion market opportunity, with long-term plans to expand into therapeutic applications, first for disorders of consciousness, but also to broader neuropsychiatric indications like dementia and TBI/stroke rehabilitation, part of a broader $400 billion total addressable market.
Momentum and Milestones
Axoft has raised over $18 million, including a $10.1 million pre-Series A round and an undisclosed grant from the state of Massachusetts to build out its manufacturing line. The company also holds exclusive licenses from Harvard University and Stanford University, protecting the foundational IP behind Fleuron.
This spring, Axoft made Fleuron available to external partners, marking its commercial debut as a next-generation material platform for research and industrial use.
“Fleuron is not just for Axoft,” said Le Floch. “It’s already being used by academic and industrial teams around the world.”
What’s Next for Axoft?
With its first clinical milestone achieved and regulatory pathways advancing, Axoft is preparing to raise a $40 million Series A to support its IDE application and premarket submission with the FDA.
“Our product roadmap starts with disorders of consciousness and scales toward treating complex neurological conditions,” Le Floch shared. This includes two FDA-classified devices: a Class II “read” system for disorders of consciousness, expected to enter the market in 2028, and a Class III “read and write” platform, projected for 2032, targeting broader indications such as dementia and TBI/stroke rehabilitation.
“We’ve moved out of the research phase,” Le Floch added. “Now it’s about bringing this into the clinic.”
The LSI Effect
Axoft made its debut at LSI USA ‘25, where Le Floch presented the company’s first human data just weeks after completing the study.
“It truly felt like the place to be for medical devices,” he said. “I met investors I’d been trying to reach for months—all in one place.”
The team also made valuable connections with other founders, advisors, and strategics. “Startups should try the hard things,” Le Floch reflected. “And when you meet others doing the same, it pushes you forward.”
17011 Beach Blvd, Suite 500 Huntington Beach, CA 92647
714-847-3540© 2026 Life Science Intelligence, Inc., All Rights Reserved. | Privacy Policy