Life Science Intelligence’s Weekly Medtech Pro Review provides a preview of select market data and startups covered in the full Medtech Pro Platform, which is a comprehensive market intelligence solution for medtech executives. As a preview of the types of content found on Medtech Pro, the Weekly Medtech Pro Review will cover select market data, procedure volumes, and startups.
Click here to learn more or subscribe to the full Medtech Pro platform.
Medtech Market Snapshot – Ischemic Stroke Treatment Devices
According to the WHO, stroke is the second leading cause of death globally. Ischemic stroke is the most common type of stroke, accounting for 87% of all strokes. Administration of tissue plasminogen activator (r-tPA) is considered the gold standard for the treatment of ischemic stroke. While tPA is the preferred treatment, it is estimated that nearly 69% of stroke patients are ineligible to receive tPA because of delayed hospital presentation. Endovascular therapies have become a significant alternative/complimentary option to restore blood flow in ischemic stroke patients. Types of endovascular therapies used to treat ischemic stroke include carotid stents and catheter-based, mechanical removal devices (e.g. stent retrievers, thrombectomy, and aspiration).
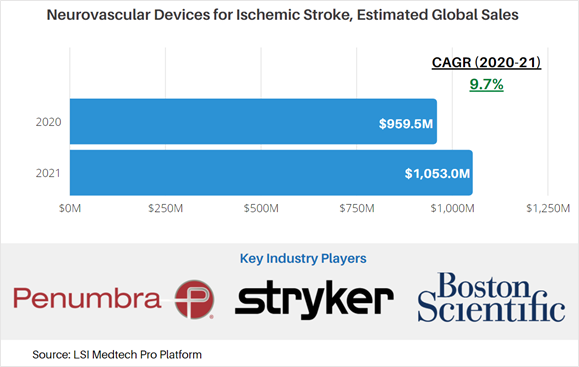
According to market data from LSI, global sales attributable to neurovascular devices for ischemic stroke treatment were approximately $959.5M in 2020. The market for these products is projected to increase at a CAGR of 9.7% to $1,053M in 2021.
Products included under LSI’s assessment of the global market for ischemic stroke treatment devices include neurovascular guidewires, microcatheters, carotid stents, aspiration and thrombectomy catheters, and stent retrievers.
For more market data on the global ischemic stroke devices market, as well as other medtech markets, visit LSI’s Medtech Pro platform.
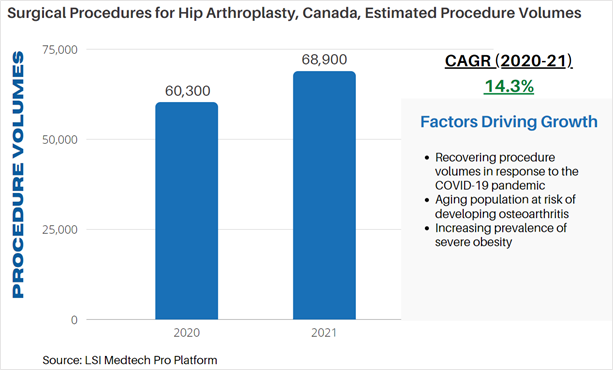
Surgical Procedure Volumes – Hip Arthroplasties in Canada
Life Science Intelligence tracks surgical procedure volumes across 37 countries and 12 major surgical markets (e.g. orthopedics, ENT, aesthetics). This week, our featured surgical procedure volume is for hip arthroplasties in Canada. Hip arthroplasty involves the replacement of parts of the joint of the hip with artificial implants. According to Pivec et al, over 90% of hip arthroplasties are performed to treat symptomatic osteoarthritis. The procedure can be performed through open or minimally invasive surgery. Surgeons must consider numerous factors to determine the surgical approach and whether a partial (ball) or total (ball and socket) replacement is necessary.
According to LSI’s Surgical Procedure Volumes database there were an estimated 60,300 hip arthroplasties performed in 2020 in Canada. Approximately 91% of hip arthroplasties performed were primary arthroplasties. The COVID-19 pandemic significantly impacted orthopedic surgeries globally due to restraints on the services and resources typically required for patients receiving orthopedic surgery – particularly with regards to available hospital beds. The average length of stay for a total hip replacement is approximately 4 days. These limitations significantly impacted the total volume of orthopedic procedures being performed, with many procedures being rescheduled or cancelled. A significant recovery in procedure volumes has taken place as countries have adapted to the impact of the pandemic. In 2021, an estimated 68,900 hip arthroplasties are projected to be performed in Canada.
Startup Spotlight

To see Aqua Medical’s presentation at the LSI Emerging Medtech Summit, click here.
Aqua Medical has developed an ablation therapy system for the treatment of prevention of gastrointestinal diseases.
Using high-temperature water vapor created by a RF generator, Aqua Medical’s Radio Frequency Vapor Ablation (RFVA) system ablates diseased tissues through an endoscopic approach. According to the company, the RFVA procedure will improve upon limitations of current ablation technologies (e.g. Medtronic’s RF Barrx System) which are unwieldly and typically require multiple procedures to treat GI diseases. The RFVA system received FDA 510(k) clearance in May 2021 for the treatment of GI tract diseases in adults.
Following the receipt of FDA clearance, Aqua Medical announced that it had closed a Series A funding round in which the company raised $15.5M.
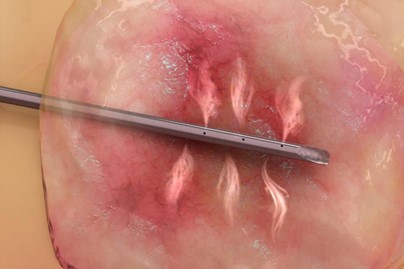
Figure 1: Example of Aqua Medical’s RFVA System ablating a pancreatic cyst
With an initial focus on GI tract diseases such as Barrett’s esophagus, the company plans to expand into treating type 2 diabetes and precancerous pancreatic cysts. Approximately 60 to 70 million Americans live with some form of GI disease, with as many as 50 million patients visiting ambulatory care centers each year due to GI diseases.

Life Science Intelligence is proud to announce that Day Zero Diagnostics will be a presenting at the 2022 Emerging Medtech Summit in Dana Point, California.
Boston-based Day Zero Diagnostics is using whole genome sequencing to change healthcare’s understanding of infectious diseases and antibiotic resistance. The company’s mission is to help healthcare providers rapidly identify the species and antibiotic resistance profiles of infections to improve the management of infectious disease.
The company uses culture-free, sequencing-based diagnostics that analyze blood samples using two computational algorithms and a proprietary, large-scale microbial database. The company’s Keynome ID is a proprietary algorithm that identifies bacterial species found within a sample at concentrations as low as ICFU/mL. Keynome g-AST is the company’s machine learning algorithm which tests the antibiotic resistance and susceptibility profile of pathogens found within a sample. Day Zero Diagnostics platform enables simultaneous pathogen and resistance testing to expedite care delivery, reduce utilization of ineffective antibiotics, and lower mortality.
Day Zero Diagnostics also offers a rapid outbreak detection service, epiXact, to help hospitals confirm outbreaks of hospital acquired infections (HAIs). Using single-nucleotide polymorphism (SNP) comparisons of the whole genome, Day Zero helps hospitals rule out or confirm the presence of HAI outbreaks in less than 2 days.


OneProjects is developing a cardiac imaging solution to improve the treatment of cardiac arrhythmias and atrial fibrillation (AF). Cardiac ablation is a complex procedure that may require multiple procedures to treat persistent forms of atrial fibrillation. Some estimates suggest that recurrent AF occurs in 20% to 40% of patients.
Verafeye is a connected platform technology that will offer physicians a comprehensive solution for imaging, pre-ablation planning, and treatment for cardiac arrhythmias and AF. The Verafeye system utilizes a catheter-based approach powered by machine learning to capture 360-degree images of the heart in real-time.
The company plans to expand the Verafeye platform beyond the treatment of arrhythmias and AF. Complex cases of cardiovascular disease could benefit from a comprehensive platform, like Verafeye, that offers pre-surgical planning, real-time imaging, and navigation.
Arrhythmia and AF are currently treated using electrophysiology navigation and ablation systems from the major strategics – Abbott, Boston Scientific, Medtronic, and market leader Biosense Webster (J&J).

Schedule an exploratory call
Request Info17011 Beach Blvd, Suite 500 Huntington Beach, CA 92647
714-847-3540© 2025 Life Science Intelligence, Inc., All Rights Reserved. | Privacy Policy