Life Science Intelligence’s Weekly Medtech Pro Review provides a preview of select market data and startups covered in the full Medtech Pro Platform, which is a comprehensive market intelligence solution for medtech executives. As a preview of the types of content found on Medtech Pro, the Weekly Medtech Pro Review will cover select market data, procedure volumes, and startups.
Click here to learn more or subscribe to the full Medtech Pro platform.
Medtech Market Snapshot – Endoscopic ENT Devices
Endoscopic surgery is a mainstay for the treatment of ear, nose, and throat conditions. Types of endoscopic ENT devices include balloon dilation catheters and endoscopes/bronchoscopes that come in rigid and flexible designs. While the COVID-19 pandemic negatively impacted total sales of endoscopic devices for ENT surgery, LSI projects that global sales will increase from $1.8B in 2020 to approximately $2.0B in 2021 – a 9.6% growth rate largely attributed to recovering procedure volumes.
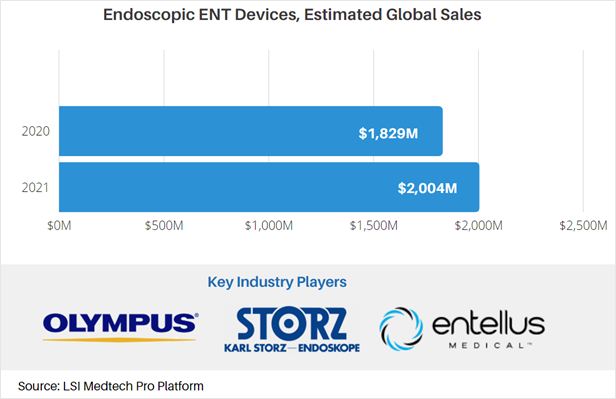
For more market data on the global endoscopic ENT devices market, as well as other medtech markets, visit LSI’s Medtech Pro platform.
Trends in Surgical Procedure Volumes
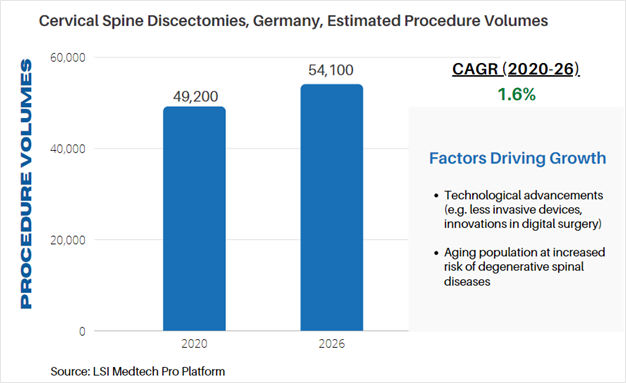
Life Science Intelligence tracks surgical procedure volumes across 37 countries and 12 major surgical markets (e.g. orthopedics, ENT, aesthetics). This week, our featured surgical procedure volume is for cervical spine discectomy procedures in Germany. Discectomies are commonly performed when a disc in the spine is compressing or irritating nearby nerves. The procedure is often used to relieve pain. According to LSI’s Surgical Procedure Volumes database there were an estimated 49,200 of these procedures performed in 2020, which represented a significant decline from estimated procedure volumes in 2019. Non-elective spine surgeries were cancelled due to the COVID-19 pandemic, but are projected to rapidly recover in 2021, and stabilize is years to follow. By 2026, LSI projects that 54,100 cervical discectomies will be performed in Germany.
Startup Spotlight

To see a CytoVeris’ presentation at the LSI Emerging Medtech Summit, click here.
CytoVeris is dedicated to improving surgical outcomes in cancer surgeries through a combination of advanced optical imaging and artificial intelligence (AI). The Connecticut-based startup is part of the growing digital surgery movement aimed at redefining how surgery is performed.
The company’s TumorMAP system is a multi-spectral imaging system to assess tissue during surgical procedures and identify cancerous tissue. At the heart of the company’s TumorMAP system is OncoSIGHT AI, a proprietary algorithm that is designed to empower surgeons with real-time visual insights that make cancer surgery smarter and safer. Initially the system is being evaluated for breast cancer, with bladder and liver cancer indications currently being assessed in the pre-clinical research stage.
The company is currently collaborating with Yale University to build and validate its OncoSIGHT platform. CytoVeris has currently raised $8M in total funding from two rounds of fundraising.
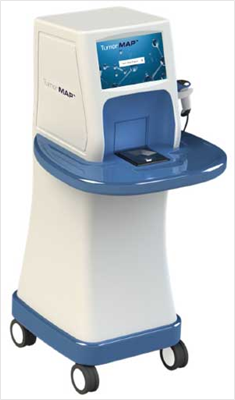
Figure 1: CytoVeris TumorMAP imaging system

Life Science is proud to announce that Obsidio will be a presenting at the 2022 Emerging Medtech Summit in Dana Point, California.
Obsidio is a pre-clinical biomaterials company developing novel solutions for embolotherapy. The company’s biomaterial will purportedly offer significant advantages over current products for transcatheter embolization.
The company’s biomaterial is being assessed for multiple applications, include interventional oncology, vascular embolization, and as a wound hemostat. The nanocomposite hydrogel technology is shear-thinning, which allows the material to become a “soft solid” after it has been forcefully injected, without relying on polymerization or precipitation as other embolic biomaterials typically require. Furthermore, the biomaterial can be loaded with other biologic agents to enable localized and sustained drug delivery.
To date the company has raised $4.8M in funding. As the company is still in the pre-clinical stage, funds have been used to develop and evaluate its hydrogel technology in multiple applications, including neurovascular, peripheral and tumor embolization.

MedRhythms is combining music, sensors, and software to create a digital therapeutics platform that can be used to help patients suffering from neurological disorders.
The mechanism of action for the company’s platform is Rhythmic Auditory Stimulation (RAS), which directly stimulates the neural circuitry that controls movement. Through a process known as “entrainment”, the auditory and motor systems of the brain are linked, resulting in enhanced neuroplasticity and improved functional outcomes. The company has a proprietary process that prescreens and adapts music in real-time to therapeutically activate songs – creating rhythmic cues that enhance the auditory-motor coupling while supporting user-preferred content.
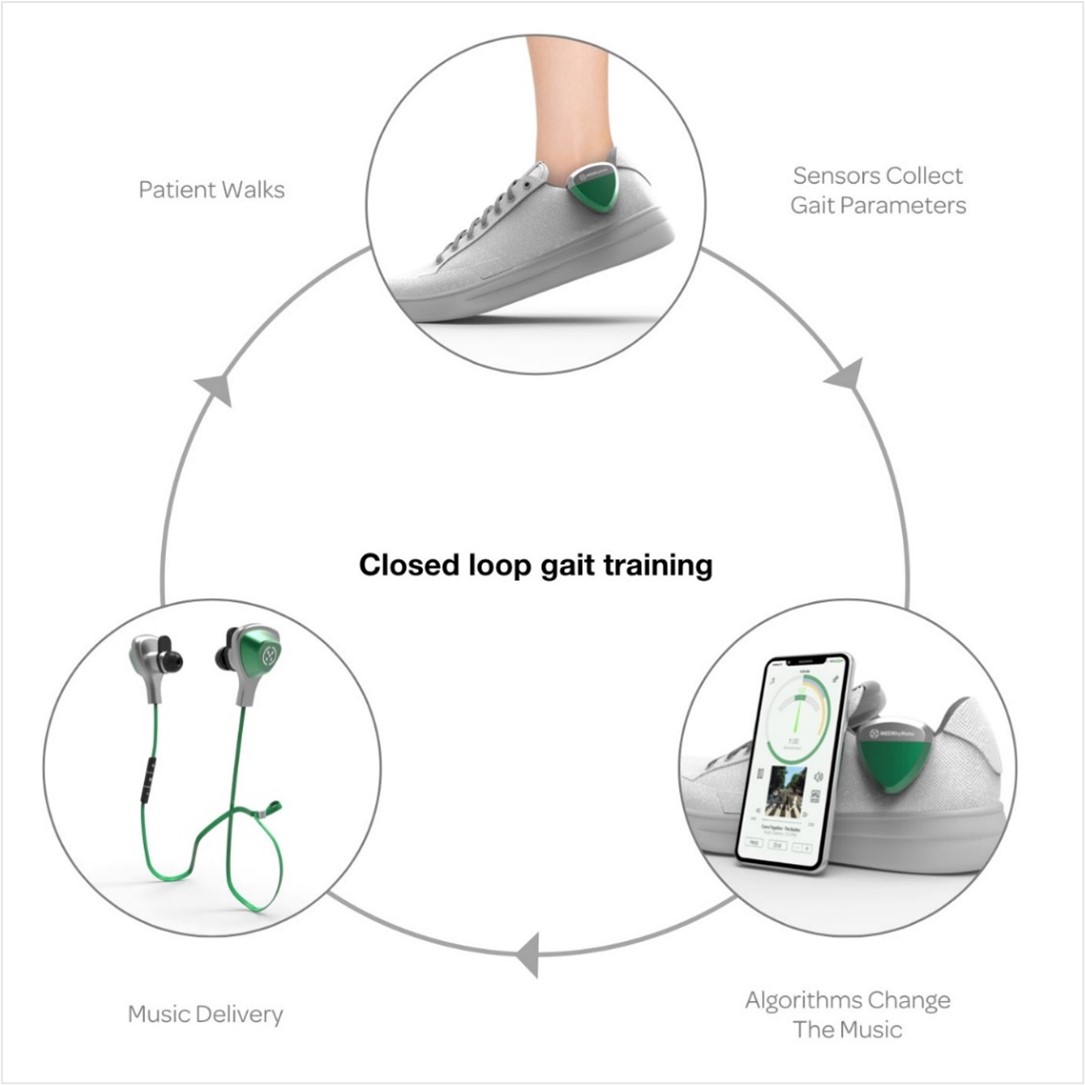
Figure 2: MedRhythms’ Rhythmic Auditory Stimulation (RAS) digital therapeutic platform
The company has received Breakthrough Device designation from the FDA for its digital therapeutic for the treatment of walking deficits caused by stroke. Currently the company is conducting a randomized control trial (RCT) to evaluate the effectiveness of the digital therapeutic in improving stroke-induced walking impairments.
Beyond stroke, the company’s therapeutic pipeline for its platform includes multiple sclerosis, Parkinson’s disease, Alzheimer’s disease/cognitive impairment, and fall prevention.
In late July 2021, MedRhythms announced that it raised $25M from a Series B round. Funds from the round will allow the company to hire key personnel, support commercialization for its lead digital therapeutic, and aid in the development of new digital therapeutics. MedRhythms has raised approximately $31M in total funding.

Schedule an exploratory call
Request Info17011 Beach Blvd, Suite 500 Huntington Beach, CA 92647
714-847-3540© 2025 Life Science Intelligence, Inc., All Rights Reserved. | Privacy Policy