
Under the direction of CEO Tim Tobin, Planatome is introducing semiconductor-level precision to surgery. By applying a nano-polishing process adapted from chip manufacturing, Planatome has created a patented technology platform that dramatically improves the performance of surgical cutting instruments—delivering faster healing, less scarring, and major economic value.
Origin Story
Planatome’s roots begin not in a medtech incubator but in the semiconductor world.
Co-founded by Tim Tobin in 2017 as a spinout of Entrepix—a semiconductor company he also led—Planatome was born from a strategic brainstorm. “Our CFO asked, ‘What other industries would pay for what we’ve built?’” said Tobin. Medical was the first to stand out. “We realized we could apply this polishing process to surgical instruments—and that it could make a huge difference in clinical healing.”
After selling Entrepix to Amtech Systems in 2023, Tobin fully spun out Planatome as an independent company focused on the medtech market.
The Current Landscape
Surgical cutting instruments haven’t changed much in a century. Most are still manufactured with diamond grit grinding wheels that leave microscale serrations—imperfections that traumatize tissue and delay healing. “Even the best blades on the market look like medieval torture devices at high magnification—all jagged and serrated that unnecessarily rip and tear tissue,” Tobin said. “That’s as good as it gets; everything else looks worse than that.”
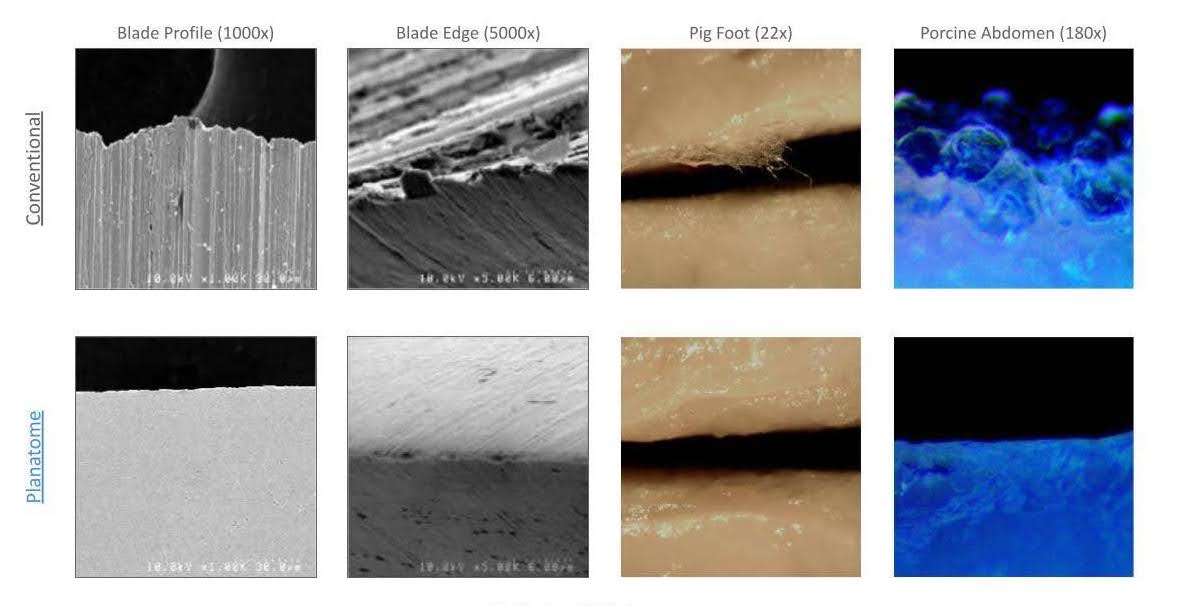
Planatome’s approach: license a patented polishing process to leading OEMs. Instead of reinventing the instrument, they enhance existing designs—scalpels, scissors, robotic blades—with a final nano-polish step that smooths the surface to near-atomic perfection.
This approach has two advantages. First, it gives Planatome a capital-efficient licensing model. Second, it’s plug-and-play for large-scale partners. “We’re not trying to commercialize the products ourselves,” said Tobin. “We just want to show the differentiation, then license the technology to the big players.”
Inside the Innovation
At the heart of Planatome’s platform is a single step: ultra-smooth polishing of the blade surface.
This subtle shift yields outsized clinical impact:
- 90% wound closure in 72 hours vs. 10% for conventional blades
- 86% reduction in hypertrophic and keloid scarring
- 50–60% lower collagen deposition, 8x reduction in TGF-beta, and 2.5x lower macrophage density
“Just by smoothing the blade edge, we reduce inflammation, accelerate healing, and minimize adverse scarring—especially in high-risk immunocompromised patients like those with diabetes,” Tobin explained.
In a preclinical study, diabetic rats treated with Planatome blades healed better than healthy rats treated with conventional ones. “The healing response was better in every measurable phase: inflammation, proliferation, and maturation,” said Tobin.
Progress and Milestones
Planatome’s licensing model is gaining momentum across multiple verticals, including its biggest opportunity: robotic-assisted surgery.
“Scalpel blades, robotic scissors, laparoscopic tools—anything that cuts can be improved with our technology,” said Tobin. In robotic platforms, blade durability matters. “With our polish, robotic scissors can last many fold longer. That’s a huge financial lever.”
To date, Planatome has raised $8 million from friends, family, and surgeon investors. The team is in active discussions with leading OEMs and expects its first licensing deals to close in early 2026. “We’re just getting started,” said Tobin. “The market is massive, and our process adds less than a dollar per unit. There’s room for everyone to win.”
Join Us at LSI Europe ‘25
Tobin has been selected to present at LSI Europe ’25 (September 7–11) in front of hundreds of global medical technology companies. Join us in welcoming him to the event in London, where he will share the latest updates on Planatome’s technology and development.

Schedule an exploratory call
Request Info17011 Beach Blvd, Suite 500 Huntington Beach, CA 92647
714-847-3540© 2026 Life Science Intelligence, Inc., All Rights Reserved. | Privacy Policy










