Overview
Valued at ~$1.59 billion in 2023, the neurovascular devices for ischemic stroke market is
projected to reach ~$2.04 billion by 2028, increasing at a CAGR of 5.1% over
the 2023-2028 forecast period. This Market Snapshot is part of LSI’s Market Intelligence platform, your
one-stop-shop for global medtech market sizing and analysis, procedure volume data, startup company- and
deal-tracking, curated insights, and more.
An ischemic stroke occurs when blood flow to the brain is blocked by a clot or thrombus, preventing the brain
from receiving oxygen and nutrients. Other causes of ischemic stroke include stenosis and embolism.
Transcatheter devices are increasingly being utilized to treat and prevent ischemic stroke. These devices
complement tissue plasminogen activator (tPA), the gold standard for treating ischemic stroke, and other
thrombolytic drugs such as tenecteplase, reteplase, and streptokinase.
Products included within the scope of this analysis include:
Neurovascular microcatheters, guidewires, guide catheters, and sheaths
Neurovascular stents
Thrombectomy devices
Stent retrievers
Aspiration devices
This Market Snapshot is intended to provide a high-level overview of the global market for neurovascular devices
for ischemic stroke, with key insights into:
Unit volumes from 2023 to 2028
Market forecasts from 2023 to 2028
Market insights
Competitive landscape analysis of major competitors
Insights into key market events for strategic and startups
Neurovascular Devices for Ischemic Stroke Market Snapshot Summary
| Snapshot Aspect | Data and Details |
| Base Year for Estimate | 2023 |
| Forecast Period | 2023 - 2028 |
| Market Size in 2023 | $1.59 billion |
| CAGR | 5.1% |
| Projected Market Size in 2028 | $2.04 billion |
Neurovascular Devices for Ischemic Stroke Market Insights
The neurovascular devices market for ischemic stroke is growing rapidly, driven by the increasing incidence of
ischemic stroke, which accounts for approximately 62.3% of all strokes globally. Devices for treating or
preventing ischemic stroke—including carotid stents, neurovascular thrombectomy devices, and aspiration
devices—were introduced more recently than devices
for hemorrhagic stroke treatment and prevention, such as embolic coils.
Modalities such as carotid stenting are used both to treat patients who have suffered a stroke to prevent
recurrence and for stroke prevention by revascularization of stenotic cerebral vessels. Mechanical thrombectomy
devices are used to remove blood clots from cerebral vessels to restore blood flow. In addition to tPA
administration, mechanical thrombectomy is a recommended approach for the management of acute stroke,
particularly in large vessel occlusions that typically respond poorly to tPA.
Epidemiological factors are the primary driving force behind unit volume and market growth. For example, trends
in obesity, sedentary lifestyles, and smoking are increasing the at-risk population for ischemic stroke, fueling
the demand for neurovascular devices for ischemic stroke and contributing to overall market growth.
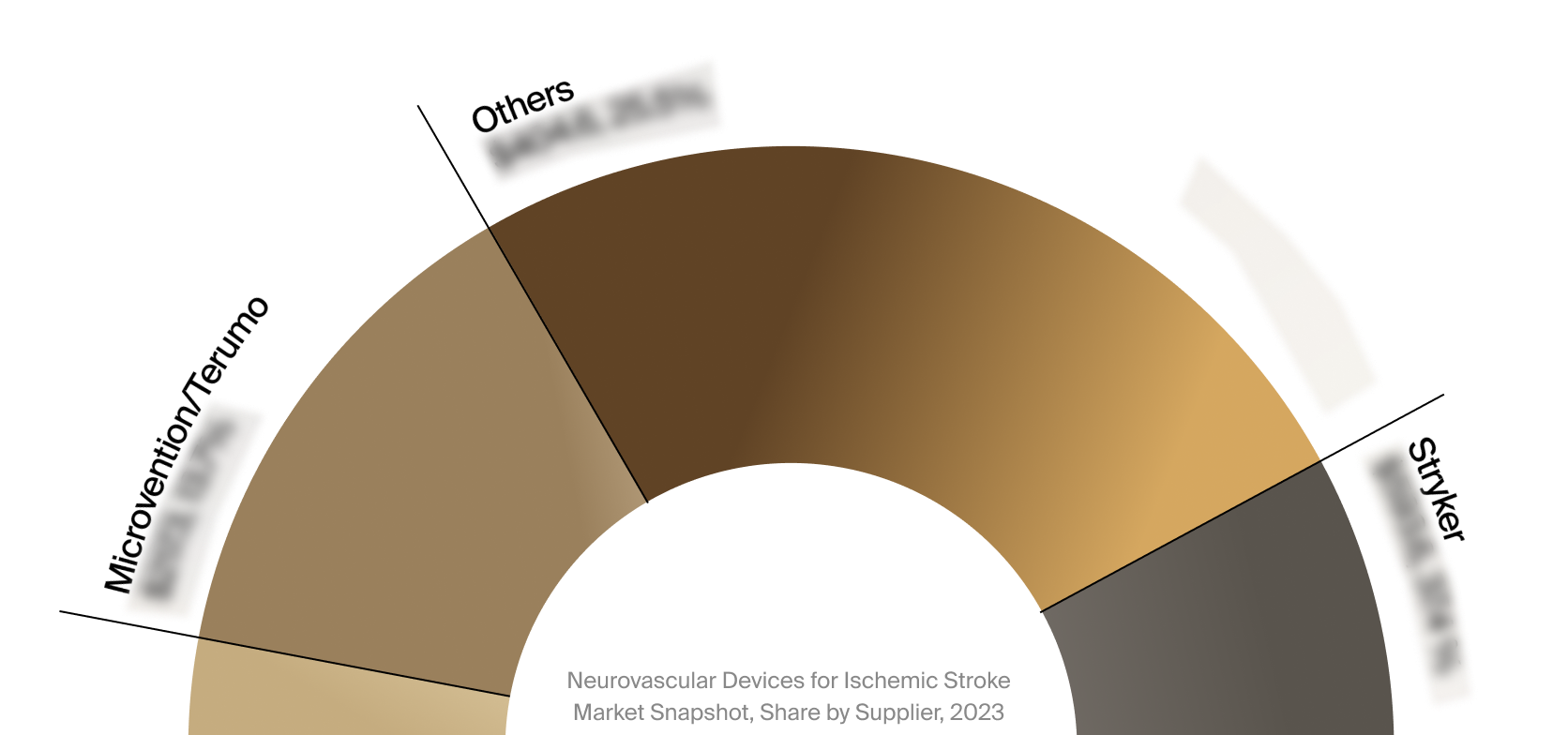
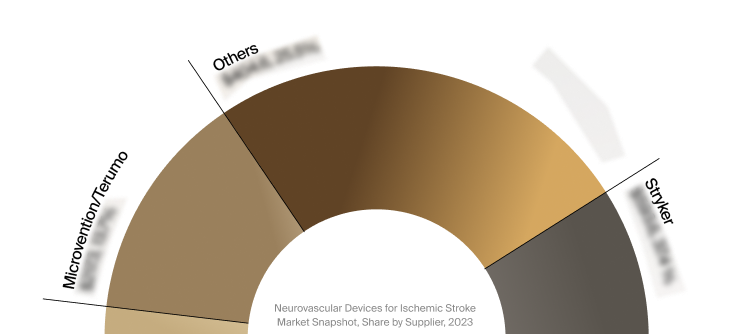
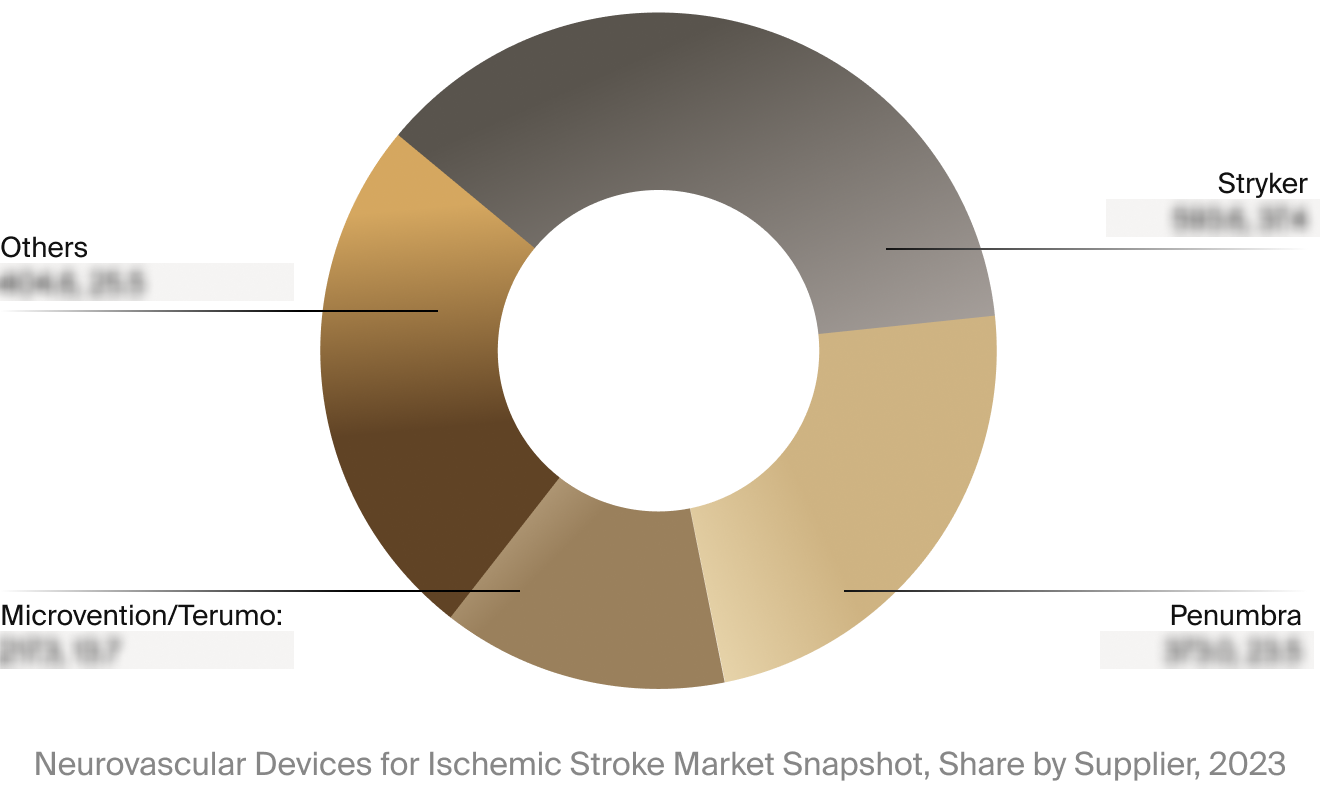
Competitive Landscape
The full Market Snapshot includes a robust analysis of the competitive landscape for the neurovascular devices
for ischemic stroke market. This includes estimated market revenue and market share for key players, such as
Stryker, Penumbra, and MicroVention/Terumo.
Select Market Events
| Company | Date | Type | Event |
|
MicroVention/Terumo
|
6/2032 | Product Launch | Microvention announced the U.S. commercial release of its ERIC Retrieval Device for treating ischemic stroke. |
|
Penumbra
|
6/2024 | Product Launch | Penumbra announced the European launch of its BMX81 and BMX96 for managing ischemic and hemorrhagic stroke. |
Key Companies Covered
Abbott
Cerenovus
Cordis
InNeuroCo
Johnson & Johnson
MicroPort Scientific
MicroVention
Penumbra
Rapid Medical
Stryker
Terumo