Overview
Valued at ~$10.0 billion in 2023, the interventional cardiology devices market is projected to
reach ~$12.4 billion by 2028, increasing at a CAGR of 4.3% over the 2023-2028
forecast period. This Market Snapshot is part of LSI’s Market Intelligence platform, your
one-stop-shop for global medtech market sizing and analysis, procedure volume data, startup company- and
deal-tracking, curated insights, and more.
The interventional cardiology devices market encompasses products employed in the diagnosis and treatment of
cardiovascular diseases, such as heart failure and severe atherosclerosis.
Products included within the scope of this analysis include:
Coronary stents
Coronary catheters (excluding diagnostic catheters)
Coronary guidewires and introducers
Intravascular ultrasound (IVUS) catheters
Coronary guide catheters
This Market Snapshot is intended to provide a high-level overview of the global market for interventional
cardiology devices, with key insights into:
Unit volumes from 2023 to 2028
Market forecasts from 2023 to 2028
Market insights
Competitive landscape analysis of major competitors
Insights into key market events for strategic and startups
Interventional Cardiology Devices Market Snapshot Summary
| Snapshot Aspect | Data and Details |
| Base Year for Estimate | 2023 |
| Forecast Period | 2023 - 2028 |
| Market Size in 2023 | $10.0 billion |
| CAGR | 4.3% |
| Projected Market Size in 2028 | $12.4 billion |
Interventional Cardiology Devices Market Insights
The interventional cardiology devices market is on a steady growth trajectory, driven by the increasing
prevalence of cardiovascular diseases and technological advancements. The prevalence of heart and circulatory
diseases has rapidly increased since 1990, fueling incident cases of cardiovascular disease requiring
intervention. Globally, there are over 200 million prevalent cases of coronary heart disease. Demand for
advanced interventional devices continues to rise, reflecting a trend toward minimally invasive procedures that
offer shorter recovery times and enhanced patient experiences.
Despite these positive trends, the market faces pricing pressures—particularly for higher-priced devices like
drug-eluting stents (DESs). In the United States, prices for DESs have dropped from around $3,000 when the
devices were first introduced to as low as $1,150, while in China, DES prices have fallen from around $2,000 to
$100. This decline is largely attributed to the implementation of Value-Based Procurement (VBP) and an
anti-corruption campaign in China's healthcare system, which have notably impacted multinational medtech
companies. Although drug-eluting stents are now considered cost-effective in developed regions such as the
United States and Europe, prices remain under pressure in developing countries where procedure growth is most
rapid.
The market is projected to grow slightly faster than the previous year, influenced by rising inflation that
counters the downward competitive pricing pressures. Overall, while challenges related to pricing persist, the
market is expected to adapt, with prices gradually increasing in response to broader economic trends.
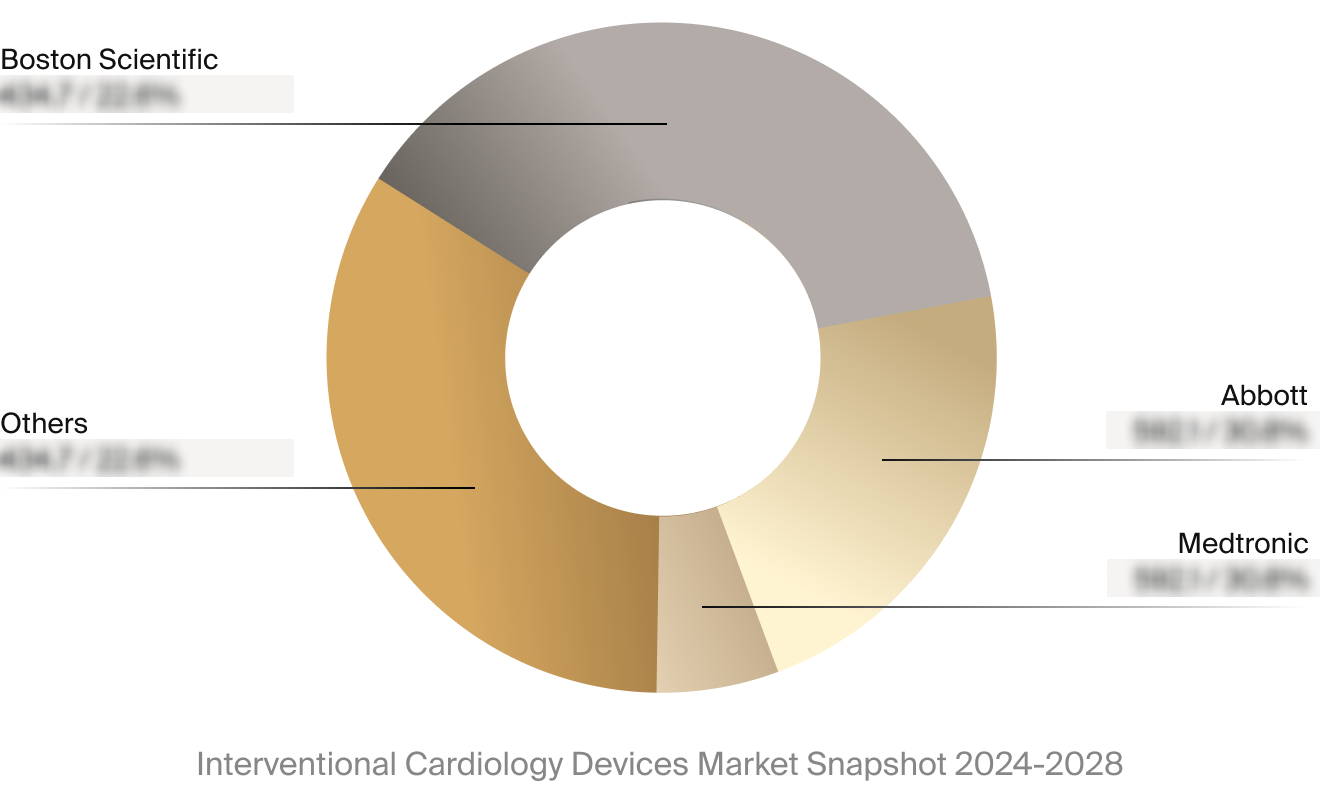
Competitive Landscape
The full Market Snapshot includes a robust analysis of the competitive landscape for the interventional
cardiology devices market. This includes estimated market revenue and market share for key players, such as
Abbott, Boston Scientific, and Medtronic.
Select Market Events
| Company | Date | Event Type | Event |
|
Boston Scientific
|
3/2024 | Regulatory | Boston Scientific received U.S. FDA approval for its AGENT™ Drug-Coated Balloon, used to treat coronary in-stent restenosis. |
|
Teleflex
|
8/2024 | Regulatory | Teleflex received U.S. FDA 510(k) clearance for its Ringer™ Perfusion Balloon Catheter for percutaneous transluminal coronary angioplasty. |
Key Companies Covered
Abbott
B. Braun
BD
Biotronik
Boston Scientific
Cook Medical
LeMaitre Vascular
LivaNova
Medtronic
Merit Medical
MicroPort Scientific
Philips
Teleflex
Terumo
For an expanded analysis of the interventional cardiology and broader cardiovascular devices market, access LSI’s
Global Medtech Market Analysis and Projections (MAP) Database.