
Under the direction of Founder and CEO Joseph C. McGinley, MD, PhD, McGinley Orthopedics is redefining orthopedic surgery with advanced, automated tools and intelligent systems designed to improve precision, safety, and efficiency in the operating room. From its flagship IntelliSenseⓇ Drill Technology to its Lever Action Plate SystemⓇ, the Wyoming-based company is introducing innovations that not only enhance patient outcomes but also reduce surgical time and cost. “We are not looking for incremental improvements but for disruptive technology that will truly impact patient care,” said McGinley.
Origin Story
The inspiration for McGinley Orthopedics came from a shared frustration among surgeons. “Renowned hand surgeon Dr. Scott Kozin was meeting with me and colleague Scott Porter after a difficult surgery,” McGinley recalled. “He remarked that a colleague repaired a wrist fracture in a young child using a traditional orthopedic drill and depth gauge, resulting in placement of a surgical screw that was too long, ultimately injuring the child’s tendon, which required a repeat surgery and tendon repair.”
That conversation sparked an idea. Drawing on McGinley’s engineering background, the trio began developing an intelligent drill that could automatically detect bone and stop before penetrating soft tissue. “Using a problem-solving approach and my background in engineering, we created a drill that could sense bone during the drilling process and auto-stop on the far side of the second cortex,” said McGinley. “The drill tells a surgeon exactly what size screw to use.”
After building and testing a prototype, they quickly realized they had created something revolutionary. “The IntelliSense Drill solves three major issues in hardware placement surgeries,” he explained. “It eliminates the need for inaccurate depth gauges and ensures the correct screw size is placed. It features an added safety mechanism that automatically stops upon breaching the bone, preventing penetration into critical structures. It also reduces the need for intraoperative X-rays to verify that screws are correct, as surgeons can be confident that they are the right length. This greatly reduces radiation exposure for both the patient and OR staff.”
Founded in 2012, McGinley Orthopedics was built around that mission: to increase patient safety and physician confidence through technological innovation. Since then, the company has developed multiple devices that advance orthopedic surgery and improve outcomes, while expanding its team and acquiring its own manufacturing facility to support growth.
The Current Landscape
McGinley Orthopedics’ first target market is the United States, where its FDA-cleared product has already gained traction among key opinion leaders in orthopedics. “We have numerous KOLs across the country championing our products,” McGinley said. “While our primary focus has been the domestic market, we’re also in discussions with international distributor networks interested in being at the forefront of medical innovation.”
The company’s devices directly address persistent issues in orthopedic hardware placement. Studies show that one in five surgical screws is improperly installed when using conventional drills. In addition, drill bit plunging, drilling too far through the bone, occurs with all surgeons in the study group, with mean plunge depths ranging between 5.6 mm and 15.8 mm, even though critical structures can be just 4 mm away. “When surgeons drill too far, they may damage blood vessels, the spinal cord, tendons, or other tissues,” McGinley explained. “These errors add up quickly in both cost and patient harm.”
Traditional orthopedic procedures also rely heavily on manual measurement and fluoroscopy. “The IntelliSense Drill’s real-time depth measurement feature eliminates the need for manual measurement, saving valuable operating room time and reducing human error,” McGinley said.
The company estimates that using IntelliSense can save hospitals $1,850 per surgery, reduce procedure time by more than 20 minutes, and potentially allow one additional surgery per day, all while reducing radiation exposure for both patients and staff. “Our mission is to improve patient outcomes while reducing costs,” said McGinley. “That’s the core of everything we design.”
Inside the Innovation
The IntelliSense Drill Technology is a simple yet transformative innovation. It utilizes real-time feedback to measure depth during drilling, automatically stopping upon breach of the second cortex. This prevents over-penetration and provides the surgeon with precise screw-length data, eliminating guesswork and reducing intraoperative imaging.
“The IntelliSense Drill provides real-time depth measurement, eliminates manual gauge errors, and reduces the need for radiation-based fluoroscopy,” McGinley said. “It tells the surgeon exactly what size screw to use, improving accuracy and confidence.”

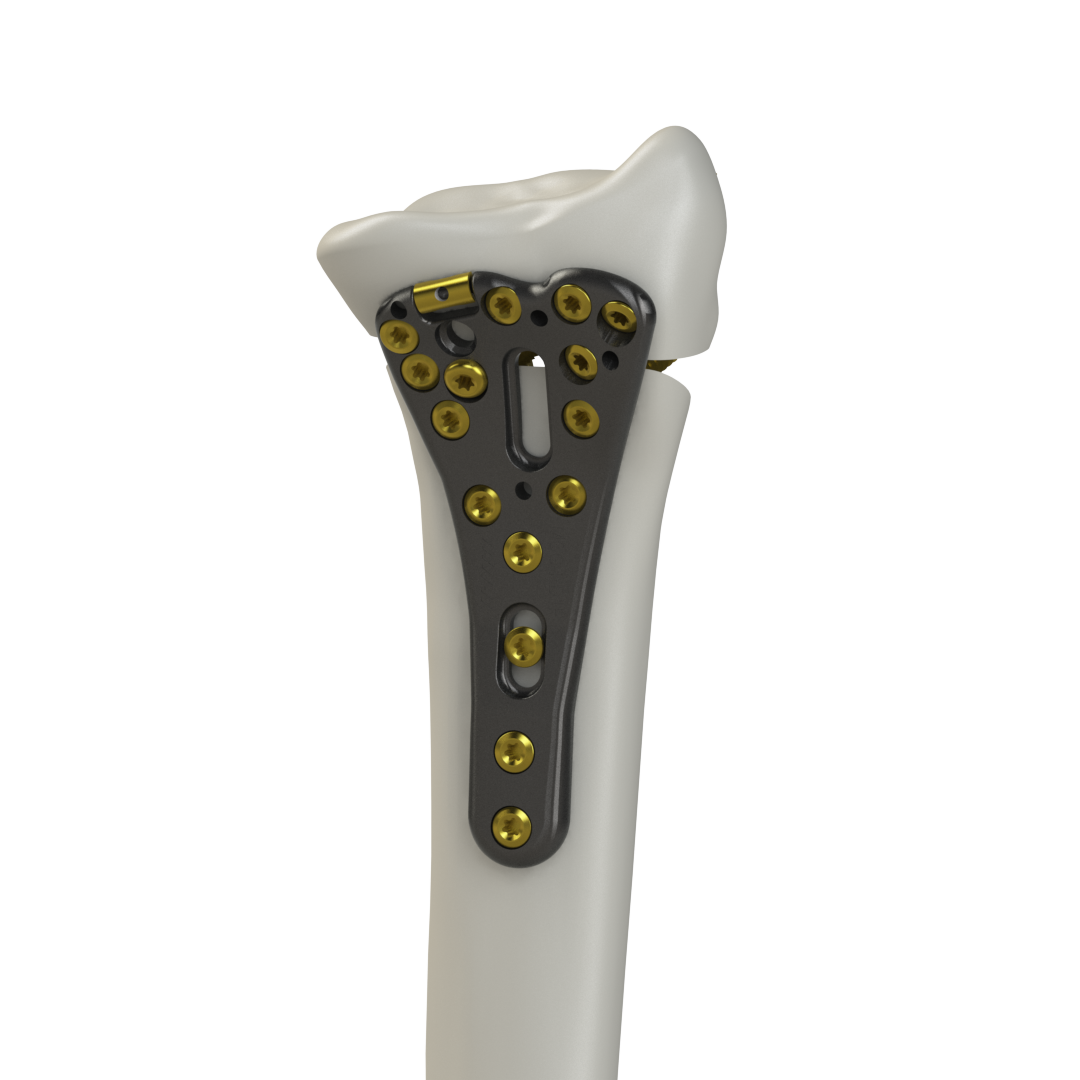
Building on the success of IntelliSense, McGinley Orthopedics launched its Lever Action Plate System, designed to improve outcomes in distal radius fracture repair. “Our Lever Action Plate System addresses major issues in the orthopedic plate systems market,” said McGinley. “It restores volar tilt and includes optional variable-angle screws with patented locking technology.”
This hybrid plate and screw system is part of a fast-growing segment of the fixation market and aims to minimize post-surgical complications. “Proper restoration of the volar tilt and articular surface is crucial for minimizing the risk of developing post-traumatic arthritis,” McGinley said. “Our Lever Action Plate System addresses this by providing proprietary control of the volar tilt.”

Progress and Milestones
Since its founding, McGinley Orthopedics has steadily expanded its product portfolio and manufacturing capabilities. “All of our products are built on the same foundation: increasing patient safety and physician confidence through technological advances in the orthopedic field,” McGinley said.
Among its upcoming products are the Freedom Nail: Ankle Fusion System, which offers improved post-fusion mobility, and RealScanNavigation™, a handheld navigation system removing the need for fiducial markers.
“RealScanNavigation integrates directly into the surgeon’s workflow,” McGinley explained. “When paired with IntelliSense technology, it provides direct contact sensing, auto-stop, and real-time navigation, effectively placing robotics into the surgeon’s hands.”
McGinley describes this as the next evolution of orthopedic surgery: accessible, affordable, safe, and easy to implement. “Ultimately, our goal is to ensure these technologies reach as many patients as possible,” he said. “That’s what drives our innovation.”
Join Us at LSI USA ‘26
McGinley has been selected to participate in an expert panel discussion at LSI USA ‘26 next March 16th–20th in front of hundreds of global medical technology companies. Join us in welcoming him to the event in Dana Point, CA, where he will share the latest updates on McGinley Orthopedics’ technology and development. To learn more about McGinley Orthopedics, go to www.mcginleyorthopedics.com.
17011 Beach Blvd, Suite 500 Huntington Beach, CA 92647
714-847-3540© 2026 Life Science Intelligence, Inc., All Rights Reserved. | Privacy Policy










