
Under the direction of Founder and CEO James Dacombe, CoMind is advancing a new era in neuromonitoring with its non-invasive optical sensing technology for measuring cerebral blood flow (CBF), intracranial pressure (ICP), and other vital brain health indicators, including cerebral autoregulation. With more than $100 million in funding and clinical trials already underway, CoMind is pushing toward FDA approval and a commercial launch in 2027.
Origin Story
The idea for CoMind was sparked by personal tragedy and a deep sense of curiosity. Dacombe founded the company at just 17 years old after witnessing the toll of neurological conditions on loved ones. “Personal experiences with family members affected by strokes and dementia inspired my interest in exploring how technology could make a difference,” he said. “Many of today’s major health challenges, including mental health disorders, Alzheimer’s, and dementia, as well as systemic issues within hospitals, can be traced to our limited understanding of how the brain works.”
Driven by the belief that brain monitoring needed a fundamental leap forward, Dacombe began assembling the foundations of CoMind, securing early support from Entrepreneur First, LocalGlobe, and the Thiel Fellowship.
The Current Landscape
CoMind is tackling a critical and underserved challenge in medicine: real-time brain monitoring. Each year, over 12 million people suffer a stroke, more than 60 million experience traumatic brain injury, and several million more undergo cardiac or vascular surgeries that can severely impact brain perfusion. Yet the standard tools for bedside neuromonitoring remain shockingly outdated.
“For ICP monitoring, the current standard is a barbaric and invasive procedure that requires drilling a hole in the skull of a patient and implanting an intracranial pressure sensor or an external ventricular drain,” Dacombe explained. “Both procedures are risky, expensive, and have high rates of adverse events such as infection and intracranial hemorrhage.”
Monitoring blood flow and autoregulation is even more limited. “There is no continuous monitor of cerebral blood flow and autoregulation available at the bedside,” said Dacombe. “As a result, surgeons and anesthesiologists lack guidance on how to target intraoperative blood pressure to maintain brain function and minimize neurological injury in individual patients. This results in a high rate of adverse events, such as postoperative cognitive dysfunction, delirium, acute kidney injury, and even ischemia and stroke in the most severe cases.”
Inside the Innovation
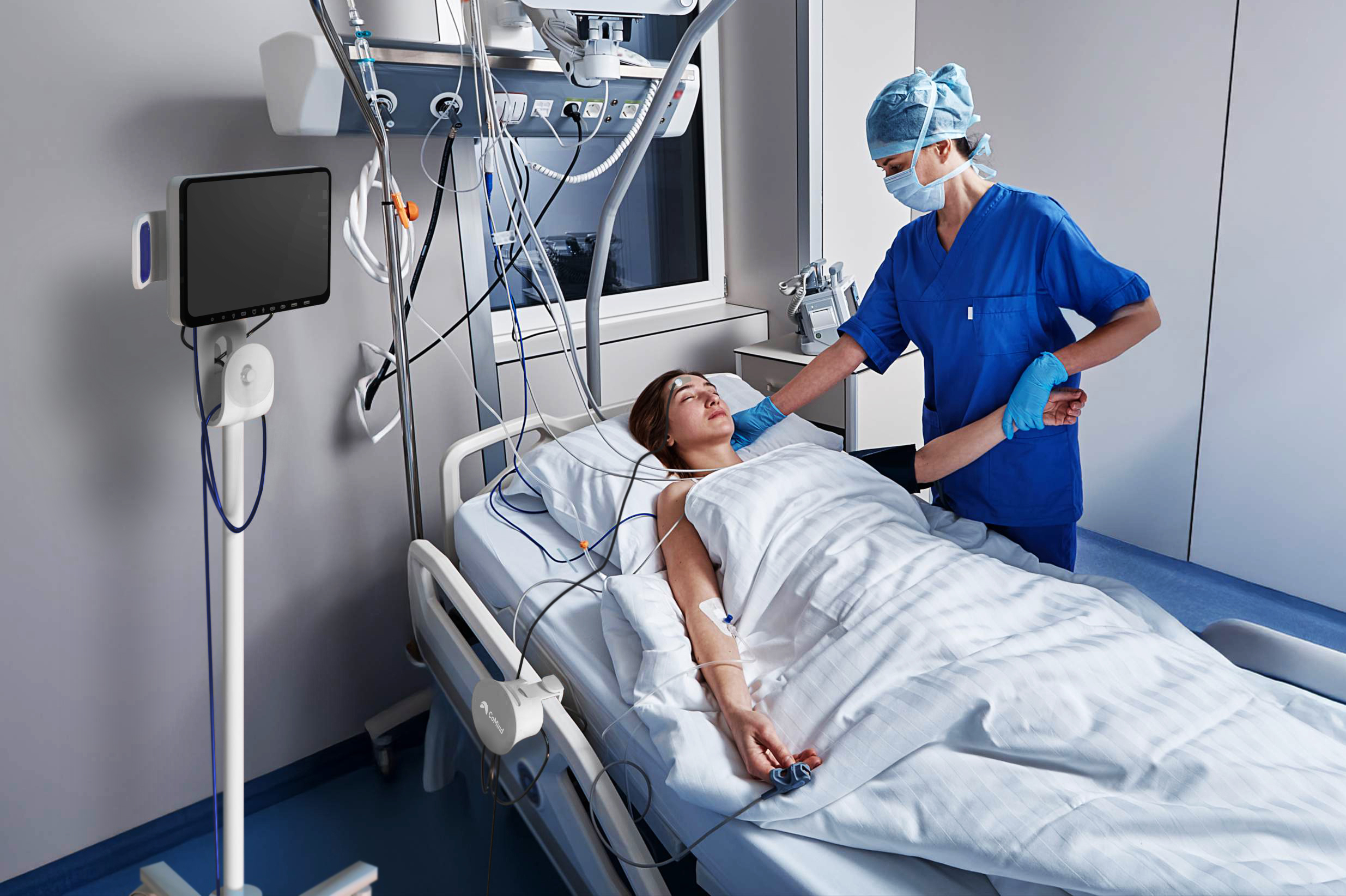
CoMind’s breakthrough lies in a novel, non-invasive optical sensing platform that leverages interferometry to measure key brain function parameters. “Our first product uses a low-power infrared laser that is swept back and forth over a narrow wavelength range extremely quickly,” said Dacombe. “The output is split in two, with one beam passing through the patient’s head and recombining with the second. This creates an interference pattern we monitor hundreds of thousands of times a second.”
Encoded in that interference pattern is an incredibly rich array of physiological information. “We can use this to determine cerebral blood flow, but also potentially to provide other critical neurophysiological parameters, including cerebral autoregulation, intracranial pressure, and, ultimately, tissue oxygenation and metabolic rate,” he said.
The device is applied as a simple forehead patch, with no need for surgical intervention. “Our technology is non-invasive, easy to use, and completely safe. It provides numerous parameters simultaneously from one sensor,” Dacombe added. “This is a huge step forward in medical photonics. We measure the time-of-flight of photons through brain tissue, which gives us superior depth discrimination and allows us to quantify absolute characteristics of the brain.”
The potential for CoMind’s technology extends beyond the ICU and operating room. The platform is being developed for integration with AI to support predictive decision-making and future alerts. “Having unlocked these signals with our optical hardware, we are beginning work on an AI platform to extract the greatest clinical value from our unique multi-dimensional data,” Dacombe said. “This platform will support personalized medical decision-making and may even be able to predict adverse neurological events before they occur.”
Progress and Milestones
CoMind’s momentum has accelerated rapidly since its founding in 2018. To date, the company has raised $102.5 million in funding, including a recent $60 million round led by Plural. “This latest round will support completion of clinical trials, full manufacturing handover, and FDA approval,” said Dacombe.
The company is currently in clinical trials and has enrolled more than 100 patients. Their first product, CoMind One, is on track for a 2027 commercial launch and is being manufactured in partnership with Benchmark Electronics.
CoMind’s vision is ambitious: to make brain monitoring as common as checking blood pressure. “By leveraging modern photonics, CoMind’s technology will create a fundamental change to the way the brain is monitored and will drive a new standard in personalized and predictive medicine,” said Dacombe.
Join Us at LSI USA ‘26
Dacombe has been selected to present at LSI USA ‘26 next March 16th–20th in front of hundreds of global medical technology companies. Join us in welcoming him to the event in Dana Point, CA, where he will share the latest updates on CoMind’s technology and development.
17011 Beach Blvd, Suite 500 Huntington Beach, CA 92647
714-847-3540© 2026 Life Science Intelligence, Inc., All Rights Reserved. | Privacy Policy










