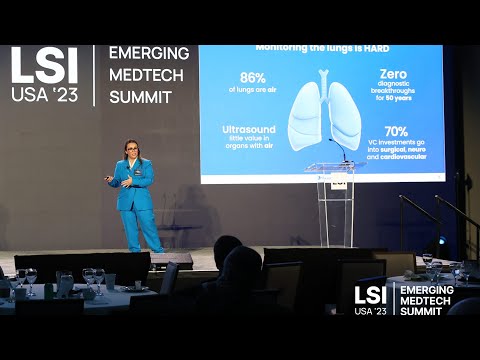
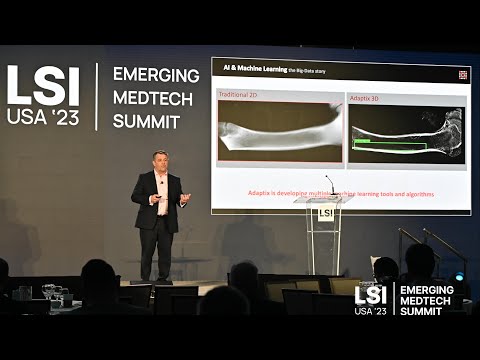
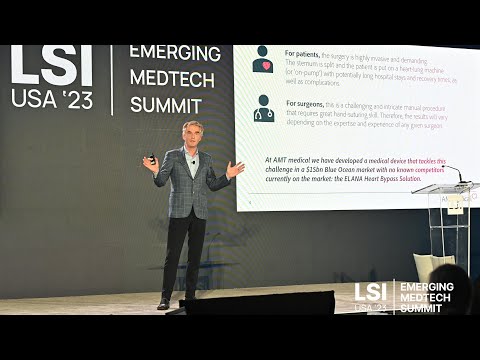
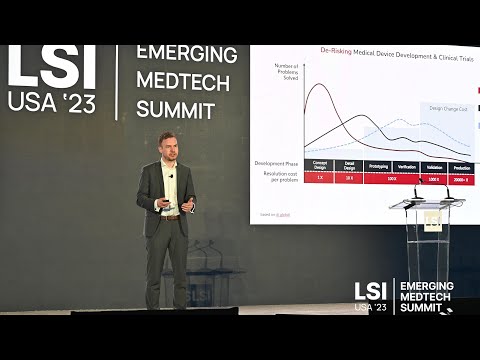
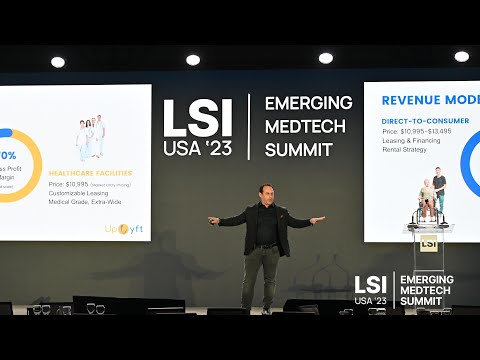
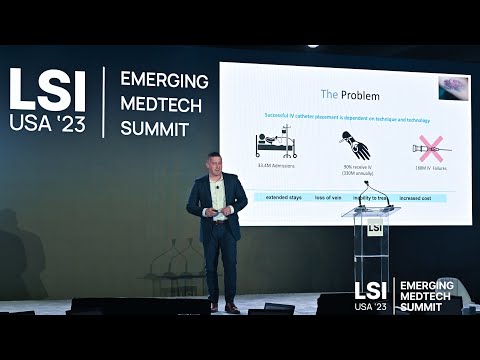
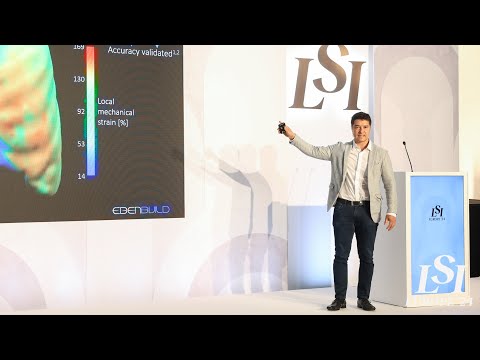
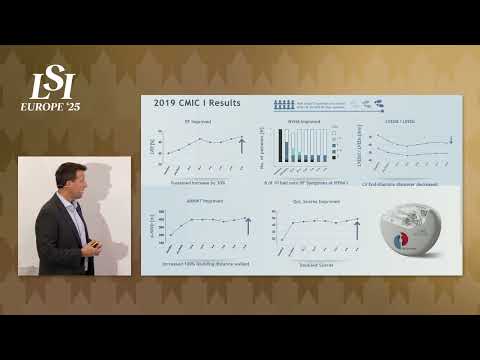
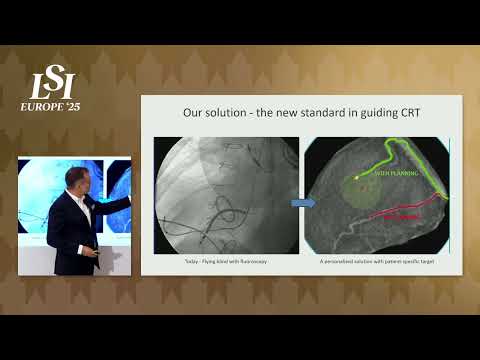
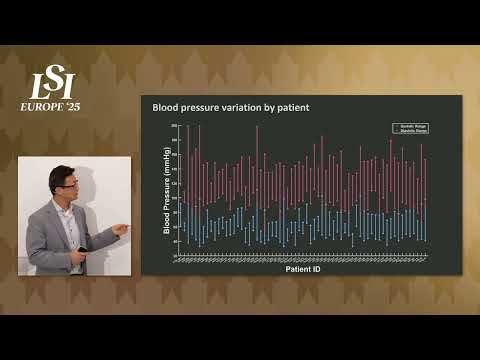
17011 Beach Blvd, Suite 500 Huntington Beach, CA 92647
714-847-3540© 2025 Life Science Intelligence, Inc., All Rights Reserved. | Privacy Policy
Subscription Includes
Global Medtech Market Analysis & Projections (MAP), 2021-2031
Published: 2023
Next Update: Q4 2024
Deliverables:



Global Medtech Market Analysis & Projections (MAP), 2021-2031
Published: 2023
Next Update: Q4 2024
Deliverables:



The Global Medtech Market Analysis and Projections (MAP) provides global market forecasts (2021 – ’31), share-by-supplier data, and analyst insights on 23 major device markets (e.g., Cardiovascular, Orthopedics, Neurovascular, In-Vitro Diagnostics) and 200+ technology subsegments. Understand major market trends and projections across the medtech industry with what medtech strategics and consulting firms have referred to as their “gold standard” for device market sizing data.
Global Surgical Procedure Volumes Dashboard, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Global Surgical Procedure Volumes Dashboard, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Global Surgical Procedure Volumes database is the single source of truth for understanding diagnostic and therapeutic procedure volumes on a global scale. With coverage on 37 countries, including the United States, Europe’s Big Five, China, India, Japan and more for 12 major procedure markets (Cardio, Ortho, General Surgery, Radiosurgery, Neuro, OB/GYN and more), this fully interactive database is designed to facilitate one-to-one analyses of procedures, countries, and regions. Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
United States Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


United States Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The United States Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for the United States. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Aesthetics, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Aesthetics, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Aesthetics Surgical Procedure Volumes Dashboard provides global, regional, and country-specific historical and projected procedure volumes forecasts from 2018 to 2029. The Aesthetics Procedures Database covers major aesthetic procedures, including: Biopsies, Punch Biopsies, Shave Biopsies, Incisional Biopsies, Lesion Removal Procedures, Curettage, electrocautery, and electrocoagulation lesion destruction, Photodestruction, Cryotherapy-based lesion destruction, Lesion Removal Procedures: Traditional and Other, Abdominoplasty, Blepharoplasty, Breast Augmentation, Breast Lift, Breast Reduction, Buttock Augmentation, Buttock Lift, Cheek Implants, Chin Augmentation, Facelift, Forehead Lift, Gynecomastia Treatment, Hair Transplantation, Lip Augmentation, Liposuction, Lower Body Lift, Otoplasty, Rhinoplasty, Thigh Lift, Upper Arm Lift, Vaginal Rejuvenation, Surgeries for Cleft Palate and Lip, Surgeries for Burn Injuries.
Cardio, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Cardio, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Cardiothoracic and Interventional Cardiology Surgical Procedure Volumes Dashboard provides global, regional, and country-specific historical and projected procedure volumes forecasts from 2018 to 2029. The Cardiothoracic and Interventional Cardiology Procedures Database covers major cardiothoracic and interventional cardiology procedures, including: CABG Surgeries Off-Pump CABG, On-Pump CABG, Valve Procedures Aortic Valve Replacements, Mitral Valve Replacements, MV Repairs, AV Repairs, PV Replacements, PV Repairs, TV Operations, Percutaneous Valvuloplasties, Percutaneous Valve Replacements, CHD Surgeries Ventricular Septal Defect Closures, Patent Ductus Arteriosus Repairs, Atrial Septal Defect Closures, Repair of Tetralogy of Fallot, Arterial Shunts Surgeries, Atrioventricular Septal Defect Repairs, Transposition of Great Artery Procedures, Anomalous Pulmonary Venous Return Repairs, Other Congenital Heart Disease Surgeries, Other Cardiothoracic Surgeries Aortic Aneurysm Procedures, Other Cardiothoracic Surgeries, Diagnostic and Therapeutic Catheterizations, Stand-Alone Diagnostic Cardiac Catheterizations, Percutaneous Coronary Interventions, Coronary Interventions Radial Approach, Coronary Interventions Femoral Approach, Heart Rhythm Procedures Conventional Pacemaker Procedures, ICD Procedures, Cardiac Resynchronization Device Procedures, Combined Defib/Resynch Device Procedures, Cardiac Ablations.
ENT, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


ENT, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Ear, Nose, and Throat Surgical Procedure Volumes Dashboard provides global, regional, and country-specific historical and projected procedure volumes forecasts from 2018 to 2029. The Ear, Nose, and Throat Surgical Procedures Database covers major ear, nose, and throat procedures, including: Tonsillectomies, Operations on the Ear, FESS, Tracheostomies, Thyroidectomies & Parathyroidectomies, Stand-Alone Adenoidectomies, Tongue Operations, Laryngeal Operations, Pharyngeal Operations, Radical Neck Dissection.
General, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


General, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The General Surgeries Procedure Volumes Dashboard provides global, regional, and country-specific historical and projected procedure volumes forecasts from 2018 to 2029. The General Surgeries Procedures Database covers major general surgical procedures, including: Cholecystectomies Laparoscopic Cholecystectomies, Open Cholecystectomies, Appendectomies, Laparoscopic Appendectomies, Open Appendectomies, Herniorrhaphies Laparoscopic Herniorrhaphies, Open Herniorrhaphies, Bariatric Surgeries Laparoscopic Bariatric, Open Bariatric, Selected Other Endoscopic General & Colorectal Procedures Esophageal (Other Endoscopic), Stomach & Intestinal (Other Endoscopic), Colorectal (Other Endoscopic), Liver (Other Endoscopic), Gallbladder & Biliary (Other Endoscopic), Pancreatic (Other Endoscopic), Other (Other Endoscopic), Other Surgeries Esophageal (Other General, non-Endoscopic), Stomach & Intestinal (Other General, non-Endoscopic), Colorectal (Other General, non-Endoscopic), Liver (Other General, non-Endoscopic), Gallbladder & Biliary (Other General, non-Endoscopic), Pancreatic (Other General, non-Endoscopic), Other (Other General, non-Endoscopic).
Neuro, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Neuro, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Neurosurgery Procedure Volumes Dashboard provides global, regional, and country-specific historical and projected procedure volumes forecasts from 2018 to 2029. The Neurosurgery Procedures Database covers major neurosurgical procedures, including: Surgeries for Traumatic Brain Injuries, Ventricular & Shunt Surgeries, New Shunt Placements, Revision Shunt Surgeries, Endoscopic Third Ventriculostomies, Diagnostic Ventricular Endoscopies, Vascular Lesion Procedures, Vascular Lesion Surgeries, Vascular Lesion Coil Procedures, Pituitary Tumor Surgeries, Open Pituitary Tumor Surgeries, Endoscopic Pituitary Tumor Surgeries, Intracranial Tumor Surgeries, Open Intracranial Tumor Surgeries, Endoscopic Intracranial Tumor Surgeries, Cranioplasties, Intracranial Neurostimulation and Peripheral Nerve Procedures, Intracranial Neurostimulator Implant Procedures, Peripheral Nerve Neurostimulation Procedures, Other Peripheral Nerve Procedures.
OB/GYN, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


OB/GYN, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Obstetric and Gynecological Surgical Procedure Volumes Dashboard provides global, regional, and country-specific historical and projected procedure volumes forecasts from 2018 to 2029. The Obstetric and Gynecological Surgical Procedures Database covers major obstetric and gynecological procedures, including: Obstetrical Surgeries Cesarean Sections, Destructive Operations, Episiotomies, Other Obstetrical Surgeries, Gynecological Surgeries Colposcopies, Hysterectomies, Salpingo-Oophorectomies & Oophorectomies, Colposcopies, Salpingo-Oophorectomies & Oophorectomies, Hysterectomies, Total Hysterectomies, Subtotal Hysterectomies, Vaginal Hysterectomies, Breast Cancer Surgeries, Breast Reconstruction Surgeries, Breast-Conserving Surgeries, and Mastectomies, among other surgeries.
Ophthalmology, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Ophthalmology, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Ophthalmological Surgical Procedure Volumes Dashboard provides global, regional, and country-specific historical and projected procedure volumes forecasts from 2018 to 2029. The Ophthalmological Surgical Procedures Database covers major ophthalmological procedures, including: Cataract Surgeries, Phacoemulsification Surgeries, ICCE Surgeries, ECCE Surgeries, MSICS Surgeries, Refractive Surgeries.
Orthopedic, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Orthopedic, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Orthopedic Surgical Procedure Volumes Dashboard provides global, regional, and country-specific historical and projected procedure volumes forecasts from 2018 to 2029. The Orthopedic Surgical Procedures Database covers major orthopedic procedures, including: Hip Arthroplasties Revision Hip Arthroplasties, Hip Resurfacing Procedures, Primary Hip Arthroplasties - Total Hip, Partial Hip, Knee Arthroplasties Revision Knee Arthroplasties, Primary Knee Arthroplasties - Partial Knee, Primary Knee Arthroplasties - Total Knee, Extremity Fractures Using Hardware Extremity Fractures Using Hardware - Upper Extremities, Extremity Fractures Using Hardware - Fractures of the Femoral Neck & Shaft, Extremity Fractures Using Hardware - Other Lower Extremity, Arthroscopies Knees (Arthroscopies), Shoulders (Arthroscopies), Ankles (Arthroscopies), Feet & Toes (Arthroscopies), Others (Arthroscopies), Other Joint Arthroplasties Finger & Hand, Wrist, Elbow, Shoulder, Ankle & Foot.
Peripheral Vascular, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Peripheral Vascular, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Peripheral Vascular Surgeries Procedure Volumes Dashboard provides global, regional, and country-specific historical and projected procedure volumes forecasts from 2018 to 2029. The Peripheral Vascular Surgeries Procedures Database covers major peripheral vascular procedures, including: Arterial Procedures Thrombectomies & Thromboendarterectomies, Carotid Artery Thrombectomies, Upper Limb Artery Thrombectomies, Iliac Artery Thrombectomies, Renal Artery Thrombectomies, Lower Limb Artery Thrombectomies, Arterial Angioplasties, Carotid Artery Angioplasties, Upper Limb Artery Angioplasties, Iliac Artery Angioplasties, Renal Artery Angioplasties, Lower Limb Artery Angioplasties, Arterial Bypasses, Carotid Artery Bypasses, Upper Limb Artery Bypasses, Iliac Artery Bypasses, Renal Artery Bypasses, Aortofemoral and Aortobifemoral Bypasses, Femoropopliteal Bypasses, Femorotibeal Bypasses, Other Lower Limb Bypasses, Venous Procedures Head, Neck, & Upper Limb Thrombectomies & Thromboendarterectomies of Dialysis Access Venous Segments, Thrombectomies & Thromboendarectomies of Other Upper Limb Veins, Angioplasties of Dialysis Access Venous Segments, Angioplasties of Head, Neck and Other Upper Limb Veins, Lower Limb Venous Procedures Removals of the Saphenous Vein, Removals of Other Veins of the Lower Limbs.
Spine, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Spine, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Spine Surgical Procedure Volumes Dashboard provides global, regional, and country-specific historical and projected procedure volumes forecasts from 2018 to 2029. The Spine Surgical Procedures Database covers major spine procedures, including: Cervical Spine Surgeries Cervical Spine Decompressions, Cervical Spine Discectomies, Cervical Spine Fusions, Cervical Spine Disc Replacements, Thoracic and Deformity Procedures Thoracic Spine Decompressions, Thoracic Spine Discectomies, Thoracic Spine Fusions, Instrumented Procedures for Deformities, Lumbar Procedures Lumbar Spine Decompressions, Lumbar Spine Discectomies, Lumbar Spine Fusions, Insertion of Lumbar Interspinous Process Spacer, Lumbar Spine Disc Replacements, Vertebroplasties & Kyphoplasties Vertebroplasties, Kyphoplasties, Other Spine Surgeries.
SRS, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


SRS, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Stereotactic Radiosurgery Procedure Volumes Dashboard provides global, regional, and country-specific historical and projected procedure volumes forecasts from 2018 to 2029. The Stereotactic Radiosurgery Procedures Database covers major stereotactic radiosurgery procedures, including: Intracranial SRS Procedures, Extracranial SRS Procedures, Extracranial Spine Procedures, Extracranial Lung Procedures, Other Extracranial Procedures.
Urological, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Urological, Global Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Urological Surgeries Procedure Volumes Dashboard provides global, regional, and country-specific historical and projected procedure volumes forecasts from 2018 to 2029. The Urological Surgeries Procedures Database covers major urological procedures, including: Kidney Stone Procedures Extra Corporeal Shock Wave Lithotripsy, Percutaneous Nephro Lithotripsy, Ureteroscopies, Open Kidney Stone Procedures, BPH Procedures Transurethral Prostatectomies, Other BPH Surgeries, Prostatectomies, Nephrectomies Partial Nephrectomies, Radical Nephrectomies, Bladder Procedures Therapeutic Endoscopies, Diagnostic Endoscopies, Urethral Catheterizations of Bladder.
Global Markets for Hip Replacement Implants, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


Global Markets for Hip Replacement Implants, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


This market snapshot from LSI provides an analysis of the global market for hip replacement implants. The market for hip replacement implants includes all prosthetic devices used to replace damaged sections of the hip joint, including those used in total and semi/hemi replacement procedures.
This market snapshot is intended to provide a high-level overview of the global market for hip replacement implants, with key insights into: Procedure volumes from 2022 to 2028, Market forecasts from 2022 to 2028, Competitive landscape analysis of major competitors, Insights into key market events for strategics and startups.
Companies covered in this report include: B. Braun, DePuy Synthes, DJO Global, Exactech, Johnson & Johnson, Medacta, MicroPort Scientific, Ortho Development, OSSIS, SERF SAS, Smith+Nephew, Symbios Orthopedie, Total Joint Orthopedics, Zimmer Biomet.
Global Markets for Peripheral Vascular Guidewires, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


Global Markets for Peripheral Vascular Guidewires, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


Description coming soon.
Global Markets for Peripheral Atherectomy Catheters, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


Global Markets for Peripheral Atherectomy Catheters, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


Description coming soon.
Global Markets for Electrosurgery, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


Global Markets for Electrosurgery, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


Description coming soon.
Global Markets for Peripheral Vascular Balloons & Vena Cava Filter, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


Global Markets for Peripheral Vascular Balloons & Vena Cava Filter, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


This market snapshot from Life Science Intelligence provides an analysis of the global market for percutaneous transluminal angioplasty (PTA) balloons and inferior vena cava (IVC) filters. A PTA balloon is used in the treatment of peripheral artery disease (PAD) to open a narrowed or blocked artery. IVC filters are permanent or temporary devices to prevent the travel of thrombotic material to the lungs. These devices are frequently used in the management of patients with severe PAD. This market snapshot is intended to provide a high-level overview of the global market for PTA balloons and IVC filters, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, and insights into key market events for strategics and startups.
Companies covered in this report include: Abbott, Acotec Scientific, Adient Medical, B. Braun, BD, Biotronik, Boston Scientific, Brosmed, Cagent Vascular, Cardinal Health, CONMED, Cook Medical, Concept Medical, Cordis, Covellus, Degania Medical, iVascular, Medtronic, Merit Medical, NextStep Medical, Nipro, OrbusNeich, Orchestra BioMed, Philips, Terumo, TriReme Medical.
Global Markets for Mechanical Heart Valves, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


Global Markets for Mechanical Heart Valves, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


Description coming soon.
Global Markets for Tissue Heart Valve Replacement, 2023-2028
Published: 2023
Next Update: Q2 2030
Deliverables:


Global Markets for Tissue Heart Valve Replacement, 2023-2028
Published: 2023
Next Update: Q2 2030
Deliverables:


Description coming soon.
Global Markets for Transcatheter Mitral Valve Devices, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


Global Markets for Transcatheter Mitral Valve Devices, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


Description coming soon.
Global Markets for Femoral Closure, 2023-2029
Published: 2023
Next Update: Q2 2024
Deliverables:


Global Markets for Femoral Closure, 2023-2029
Published: 2023
Next Update: Q2 2024
Deliverables:


This market snapshot from Life Science Intelligence provides an analysis of the global market for femoral closure devices. Femoral closure devices are used to achieve hemostasis of the hole in the artery that is created to perform catheter-based cardiovascular or endovascular procedures. This market snapshot is intended to provide a high-level overview of the global market for femoral closure devices, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, insights into key market events for strategics and startups.
Companies covered in this report include: Abbott, Cardinal Health, Cardiva, Cordis, CyndRx, Endocor, Haemonetics, Morris Innovative, Rex Medical, Teleflex, Terumo, Transluminal Technologies, Vasorum, Vivasure Medical.
Global Markets for Tricuspid Valve Repair, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


Global Markets for Tricuspid Valve Repair, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


This market snapshot from Life Science Intelligence provides an analysis of the global market for tricuspid valve repair devices. Tricuspid valve repair is the preferred surgical approach for the treatment of degenerative tricuspid valve disease. The market is currently experiencing a renaissance with the introduction and development of transcatheter solutions for tricuspid valve repair and replacement. Devices covered within the scope of this analysis include tricuspid valve annuloplasty rings and transcatheter tricuspid valve repair devices. This market snapshot is intended to provide a high-level overview of the global market for tricuspid valve repair devices, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, and insights into key market events for strategics and startups.
Companies covered in this report include: Abbott, CroiValve, Edwards Lifesciences, Innoventric, Medtronic, Mitral Holdco, Mitralix, NaviGate, OrbusNeich, TriCares, Venus Medtech.
Global Markets for Percutaneous Pulmonary Valves, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


Global Markets for Percutaneous Pulmonary Valves, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


This market snapshot from Life Science Intelligence provides an analysis of the global market for transcatheter pulmonary valve devices. Pulmonary valve replacement is performed primarily for the treatment of pulmonary valve stenosis, a relatively rare disease associated with congenital heart defects. Devices covered within the scope of this analysis include transcatheter pulmonary valve implants for valve repair and replacement. This market snapshot is intended to provide a high-level overview of the global market for transcatheter pulmonary valve devices, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, and insights into key market events for strategics and startups.
Companies covered in this report include: Edwards Lifesciences, Medtronic, PolyVascular, Venus Medtech.
Global Markets for Coronary Angio Guidewires & Catheters, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


Global Markets for Coronary Angio Guidewires & Catheters, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


This market snapshot from Life Science Intelligence provides an analysis of the global market for coronary angiography catheters and guidewires. These devices are essential to perform coronary angiography, a minimally invasive medical procedure used to visualize blood flow, identify blockages and narrowing of the coronary arteries. Devices covered within the scope of this analysis include coronary angiography catheters and coronary angiography guidewires. This market snapshot is intended to provide a high-level overview of the global market for coronary angiography catheters and guidewires, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, insights into key market events for strategics and startups.
Companies covered in this report include: Abbott, AngioDynamics, B. Braun, Boston Scientific, Cardinal Health, Cook Medical, Cordis, Medtronic, Merit Medical, Philips, Teleflex, Terumo.
Global Markets for Oncology Ablation Devices, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


Global Markets for Oncology Ablation Devices, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


This market snapshot from Life Science Intelligence provides an analysis of the global market for oncology ablation catheters. These devices are used as a therapeutic modality employing energy to selectively destroy cancerous tissue. Devices covered within the scope of this analysis include radiofrequency and microwave ablation electrodes, excluding cryoablation devices, which are covered in a separate report.
The snapshot offers a high-level overview of the global market for oncology ablation catheters and guidewires, with key insights into unit volumes and market forecasts from 2023 to 2028, along with a competitive landscape analysis of major competitors and insights into key market events for strategics and startups.
Companies covered in this report include: AngioDynamics, Baylis, Boston Scientific, Canyon Medical, CAPS Medical, EDAP, Galvanize Therapeutics, Imagin Medical, Medtronic, Mermaid, Mirai Medical, Monteris, Sonablate, Stryker, TROD Medical, and US Medical Innovations.
Global Markets for ENT Devices, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


Global Markets for ENT Devices, 2023-2028
Published: 2023
Next Update: Q2 2024
Deliverables:


This market snapshot from Life Science Intelligence provides an analysis of the global market for endoscopic devices for ear, nose, and throat (ENT) surgery. These devices are used to examine and operate on the structures and tissues in the ear, nose, and throat. Devices covered within the scope of this analysis include ENT endoscopic surgical instruments, balloon sinus and dilation catheters, and rigid endoscopes and bronchoscopes. This market snapshot is intended to provide a high-level overview of the global market for ENT endoscopic surgery devices, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, insights into key market events for strategics and startups.
Companies covered in this report include: 3NT Medical, Acclarent, Conmed, Endoluxe, Entellus Medical, Intuitive Surgical, Johnson & Johnson, KARL STORZ, Medtronic, Olympus, Pentax, Pristine Surgical, Richard Wolf, Smith & Nephew, Stryker, Tympany Medical.
Global Markets for Cell Delivery Catheters, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


Global Markets for Cell Delivery Catheters, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


This market snapshot from Life Science Intelligence provides an analysis of the global market for cell delivery catheters. These devices are primarily research-use devices for the delivery of cellular therapies for the treatment of chronic diseases, such as cardiovascular disease and cancer. Devices covered within the scope of this analysis include cell delivery catheters. This market snapshot is intended to provide a high-level overview of the global market for cell delivery catheters, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, insights into key market events for strategics and startups.
Companies covered in this report include: BioCardia, Biosense Webster, Boston Scientific, Cordis, Medtronic, Mercator MedSystems, TriSalus Life Sciences, TRI Medical.
Global Markets for Urology Devices, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


Global Markets for Urology Devices, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


This market snapshot from Life Science Intelligence offers an analysis of the global market for urology and renal devices, focusing on treatments for six major conditions: End-stage renal disease (ESRD), Incontinence, Calculi (stones), Benign prostatic hyperplasia (BPH), Prostatitis, and Erectile dysfunction (ED).
The devices covered within this analysis encompass a range of tools for diagnosis, treatment, and management, including urinary bags, foley catheters, catheter insertion kits, intermittent catheters, endourology devices, BPH ablation devices, lithotripsy devices, and artificial penis devices.
The snapshot provides key insights into unit volumes and market forecasts spanning from 2023 to 2028. Additionally, it includes a competitive landscape analysis of major competitors and insights into key market events for strategics and startups.
Companies covered in this report include: 3M, Applied Medical, Astratech, Balton, Baxter Healthcare, B. Braun, BD, Boston Scientific, Calyxo, Corinth Medtech, Coloplast, EndoMed, Flume Catheter Company, Hollister, ICU Medical, Karl Storz, Medline, Medtronic, Merit Medical, Nipro, Olympus, SonoMotion, Teleflex, and Urovision.
Global Markets for External Pain Pumps, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


Global Markets for External Pain Pumps, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


This market snapshot from Life Science Intelligence provides an analysis of the global market for external pain pumps. These devices are used to provide patients with consistent, long-lasting pain relief by delivering pain medication epidurally, intravenously, or subcutaneously. Devices covered within the scope of this analysis include external infusion pumps for pain management. This market snapshot is intended to provide a high-level overview of the global market for external pain pumps, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, insights into key market events for strategics and startups.
Companies covered in this report include: Avanos, B. Braun, BioQ Pharma, Epic Health, ICU Medical, InfuTronix, Medical Flow Systems, Medipacs, MicroPort, Smiths Medical, Teleflex.
Global Markets for Ureteral Access Devices, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


Global Markets for Ureteral Access Devices, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


This market snapshot from Life Science Intelligence delves into the global market for ureteral access devices, crucial components utilized alongside ureteroscopy or percutaneous nephrolithotomy procedures. These devices facilitate dilation and create a working channel for various urologic interventions.
The snapshot aims to offer insights into unit volumes and market forecasts spanning from 2023 to 2028. Additionally, it provides a competitive landscape analysis of major competitors and key market events for strategics and startups.
Companies covered in this report include: Amecath, Applied Medical, BD, Boston Scientific, Cook Medical, Envaste, Johnson & Johnson, Mednova, Olympus, Richard Wolf, Rocamed, Teleflex, and Terumo.
Global Markets for Pelvic Floor Repair, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


Global Markets for Pelvic Floor Repair, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


This market snapshot from Life Science Intelligence provides an analysis of the global market for pelvic floor repair mesh. These products are used during surgical procedures to repair prolapse and urinary incontinence. Devices covered within the scope of this analysis include pelvic floor repair/reconstruction mesh. This market snapshot is intended to provide a high-level overview of the global market for pelvic floor repair mesh, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, and insights into key market events for strategics and startups.
Companies covered in this report include: BD, Boston Scientific, Coloplast, Cook Medical, Ethicon, Johnson & Johnson.
Global Markets for Atrial Fibrillation, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


Global Markets for Atrial Fibrillation, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


Description coming soon.
Global Markets for Neurovascular Devices Ischemic, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


Global Markets for Neurovascular Devices Ischemic, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


This report from Life Science Intelligence analyzes the global market for devices used in the treatment of ischemic stroke, focusing on solutions designed to remove occlusions in blood vessels to prevent and treat this condition.
The market snapshot offers insights into unit volumes and market forecasts from 2023 to 2028, along with a competitive landscape analysis of major competitors and key market events for strategics and startups.
Companies covered in this report include: Cerenovus, Ceretrieve, Cordis, InNeuroCo, Julier, Medtronic, MicroPort Scientific, Microvention, Penumbra, Phenox, Poseydon Medical, Route 92 Medical, Stryker, and Terumo.
Global Markets for Neuromodulation Devices, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


Global Markets for Neuromodulation Devices, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


This report from Life Science Intelligence provides an analysis of the global neuromodulation market – a thriving market for managing and treating neurological diseases, chronic pain, and other chronic conditions. Types of neuromodulation, or neurostimulation, devices include implantable and transcutaneous devices, including wearable devices. This market snapshot is intended to provide a high-level overview of the global neuromodulation market, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, insights into key market events for strategics and startups.
Companies covered in this report include: Abbott, Advanced Bionics, Alyve Medical, Axonics, Biotronik, Boston Scientific, EBT Medical, electroCore Medical, Inspire Medical, LivaNova, Medtronic, Neuromod Devices, Nevro.
Global Markets for Vertebroplasty Devices, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


Global Markets for Vertebroplasty Devices, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


This market snapshot from Life Science Intelligence offers an analysis of the global market for vertebral augmentation products, which are crucial in treating vertebral compression fractures, often caused by osteoporosis or spinal tumors. Vertebroplasty and kyphoplasty are the primary surgical interventions, and the market includes products such as vertebroplasty and kyphoplasty cement, as well as delivery devices.
The snapshot aims to provide a comprehensive overview of the global vertebral augmentation products market, with key insights into unit volumes and market forecasts from 2023 to 2028. Additionally, it includes a competitive landscape analysis of major competitors and insights into key market events for both established players and startups.
Companies covered in this report include: Halma, IZI Medical, Medtronic, Mendec, Merit Medical, RevBio, and Stryker.
Global Markets for Benign Prostation Hyperplasia Implants, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


Global Markets for Benign Prostation Hyperplasia Implants, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


This report from Life Science Intelligence provides an analysis of the global market for implants for the treatment of symptomatic benign prostatic hypertrophy/hyperplasia (BPH). These implants are reversible solutions for men suffering from lower urinary tract symptoms (LUTS) caused by prostate enlargement. This market snapshot is intended to provide a high-level overview of the global BPH implants market, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, and insights into key market events for strategics and startups.
Companies covered in this report include: Butterly Medical, Endotherapeutics, Olympus, ProArc, ProVerum, Teleflex, Urotronic, ZenFlow.
Global Markets for Cryoablation, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


Global Markets for Cryoablation, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


This report from Life Science Intelligence provides an analysis of the global market for cryoablation devices for non-cardiovascular applications. These devices are used to freeze and induce cellular damage and death for indications including cancer, dermatological conditions, peripheral vascular disease, and urological conditions. This market snapshot is intended to provide a high-level overview of the global cryoablation devices market, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, insights into key market events for strategics and startups.
Companies covered in this report include: Boston Scientific, Brymill Cryogenics, Channel Medsystems, CooperSurgical, CPSI Biotech, CryoConcepts, CryoProbe, CryoSurgery, CSA Medical, Endocare, Endocision, Grand Cryo, Ictero Medical, KryoLife, Mectronic Medical, Sedivention, Siemens Healthineers, Varian Medical Systems, Wallach Surgical.
Global Markets for Diagnostic Electrophysiology Catheters, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


Global Markets for Diagnostic Electrophysiology Catheters, 2023-2028
Published: 2023
Next Update: Q3 2024
Deliverables:


This market snapshot from Life Science Intelligence provides an analysis of the global market for diagnostic electrophysiology catheters. These devices are used to measure and map electrical activity within the heart to identify aberrant electrical activity that causes arrhythmias. LSI projects that this market will remain in a high growth phase due to improving technology for the surgical treatment of cardiac arrhythmias and atrial fibrillation (AF). This market snapshot is intended to provide a high-level overview of the global diagnostic electrophysiology catheters market, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, insights into key market events for strategics and startups.
Companies covered in this report include: Abbott, AccuPulse, Acutus Medical, BD, Biosense Webster, Boston Scientific, CardioNXT, CoreMap, Johnson & Johnson, Kardium, Medtronic, MicroPort Scientific, Stereotaxis.
Global Markets for Hernia Repair, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


Global Markets for Hernia Repair, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


This market snapshot from Life Science Intelligence (LSI) provides an analysis of the global market for hernia mesh products for the surgical repair of hernias. The global market for hernia mesh products is projected to see moderate growth throughout the forecast period covered by the analysis. While the market has been negatively impacted by ongoing lawsuits associated with product complications, the next generation of products has helped the market to recover for one of the most performed abdominal surgeries. This market snapshot is intended to provide a high-level overview of the global market for hernia mesh products, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, insights into key market events for strategics and startups.
Companies covered in this report include: Allergan, Ariste Medical, BD, Cook Medical, Deep Blue Medical Advances, Ethicon, Exogenesis, Integra Lifesciences, Johnson & Johnson, LifeCell, Medtronic, Novus Scientific, Tarian Medical, TELA Bio, TISSIUM, W.L. Gore.
Global Markets for CRM Devices, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


Global Markets for CRM Devices, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


This market snapshot from Life Science Intelligence provides an analysis of the global market for cardiac rhythm management (CRM) devices, including pacemakers, implantable cardioverter defibrillators (ICDs), and cardiac resynchronization therapy devices (CRT-Ds). These implantable devices are used to restore the natural rhythm and function of the heart that can be impaired as a result of cardiac rhythm disorders and heart failure. This market snapshot is intended to provide a high-level overview of the global CRM devices market, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, insights into key market events for strategics and startups.
Companies covered in this report include: Abbott, AtaCor Medical, BioTrace Medical, Biotronik, Boston Scientific, Cairdac, Electroducer, FineHeart, Lepu Medical, LivaNova, Medico, Medtronic, Merit Medical, Microport Scientific, Osypka Medical, Pacetronix.
Global Markets for Neurovascular Devices Hemorrhagic, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


Global Markets for Neurovascular Devices Hemorrhagic, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


This report from Life Science Intelligence provides an analysis of the global market for neurovascular devices for the treatment of hemorrhagic stroke. These solutions are used to treat intracerebral bleeds, ruptured aneurysms, and other neurovascular deformities that lead to the pooling of blood vessels that have ruptured inside and outside of the brain. This market snapshot is intended to provide a high-level overview of the global market for neurovascular devices for hemorrhagic stroke, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, insights into key market events for strategics and startups.
Companies covered in this report include: Acandis, Artio Medical, Endostream Medical, Medtronic, MicroPort Scientific, Microvention, Phenox, Penumbra, Rapid Medical, Shape Memory Medical, Stryker, Terumo, Wallaby Medical.
Global Markets for Renal Denervation, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


Global Markets for Renal Denervation, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


This market snapshot from Life Science Intelligence provides an analysis of the global market for renal denervation devices for the treatment of refractory hypertension (i.e., high blood pressure that does not respond to pharmaceuticals). The market for renal denervation devices includes radiofrequency and ultrasound catheters which are used to ablate the renal nerves. This market snapshot is intended to provide a high-level overview of the global market for renal denervation devices, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, and insights into key market events for strategics and startups.
Companies covered in this report include: Ablative Solutions, Brattea, Medtronic, Metavention, Otsuka Medical, ReCor Medical, SoniVie.
Global Markets for Upper+Lower Suture Anchors, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


Global Markets for Upper+Lower Suture Anchors, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


This market snapshot from Life Science Intelligence analyzes the global market for suture anchors used in upper and lower extremity repair and reconstruction procedures. Suture anchors play a critical role in securing soft tissue to bone, particularly for severe soft tissue tears. The market encompasses bioabsorbable, biocomposite, metallic, and PEEK suture anchors.
The snapshot aims to offer insights into unit volumes and market forecasts from 2023 to 2028, along with a competitive landscape analysis of major competitors and key market events for strategics and startups.
Companies covered in this report include: Acumed, Acuitive Technologies, aevumed, Anika Therapeutics, Arthrex, ConMed, DePuy Synthes, Johnson & Johnson, Mitek, OSSIO, Paragon 28, Responsive Arthroscopy, Riverpoint Medical, Smith & Nephew, Stryker, and Zimmer Biomet.
Global Markets for Peripheral Stents, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


Global Markets for Peripheral Stents, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


This market snapshot from Life Science Intelligence provides an analysis of the global market for biliary and peripheral vascular stents. These devices are used to maintain and restore patency to anatomical ducts or vessels that have become obstructed due to the formation of plaque, narrowing of the natural lumen, or presence of benign and malignant growths. This market snapshot is intended to provide a high-level overview of the global peripheral vascular and biliary stents market, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, and insights into key market events for strategics and startups.
Companies covered in this report include: Abbott, BD, Biotronik, Boston Scientific, Bryan Medical, Cardinal Health, Cook Medical, Cordis, Endo GI Medical, Hood Labs, Maquet, Medtronic, Merit Medical, MicroPort Scientific, Olympus, Q3 Medical, Zorion Medical.
Global Markets for Electromagnetic Navigation Systems, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


Global Markets for Electromagnetic Navigation Systems, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


This market snapshot from Life Science Intelligence provides an analysis of the global market for electromagnetic navigation systems for tracking, guiding, and positioning instruments during surgery. These systems provide surgeons and the surgical team with real-time information on the position and orientation of instruments used during open and minimally invasive surgeries. This market snapshot is intended to provide a high-level overview of the global electromagnetic navigation systems market, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, insights into key market events for strategics and startups.
Companies covered in this report include: Brainlab, Collin Medical, Elucent Medical, Fiagon, Heal Force, Joimax, Karl Storz, Medtronic, Olympus, Stryker, Veran Medical Technologies.
Global Markets for GI Endoscopy, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


Global Markets for GI Endoscopy, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


This market snapshot report from Life Science Intelligence provides an analysis of the global market for gastrointestinal (GI) endoscopic devices. These devices are used to visualize, diagnose, and surgically treat conditions of the GI tract. The market for GI endoscopic devices includes endoscopes and endoscopic instruments. This market snapshot is intended to provide a high-level overview of the global market for GI endoscopic devices, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, insights into key market events for strategics and startups.
Companies covered in this report include: Ambu, Applied Medical, Aqua Medical, Aspero Medical, B. Braun, Boston Scientific, ConMed, Endoluxe, Ethicon, Integra Lifesciences, Intuitive Surgical, IQ Endoscopes, Johnson & Johnson, Karl Storz, Medtronic, MiWendo Solutions, Olympus, Purple Surgical, Richard Wolf, Stryker, Teleflex.
Global Markets for Hemodialysis, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


Global Markets for Hemodialysis, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


This market snapshot from Life Science Intelligence provides an analysis of the global market for hemodialysis solutions. Hemodialysis is an essential treatment for patients suffering from end-stage renal disease due to kidney failure. Hemodialysis systems, catheters, and dialyzers are components integral to hemodialysis treatment. This market snapshot is intended to provide a high-level overview of the global hemodialysis solutions market, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, insights into key market events for strategics and startups.
Companies covered in this report include: Asahei Kasei, AngioDynamics, B. Braun, Baxter, Bellco, Byonyks, Diality, Fresenius, Hemoclean, Humacyte, Intermedt, Inspira Health, iRen-MEDICAL, Medivators, Merit Medical, NextKidney, Nikkiso, Nipro, Rockwell Medical, Telelfex, Terumo, Toray Medical, TVA Medical, Vantive.
Globals Markets for Cardiac Ablation, 2023-2028
Published:
Next Update:
Deliverables:
Globals Markets for Cardiac Ablation, 2023-2028
Published:
Next Update:
Deliverables:
This market snapshot from Life Science Intelligence provides an analysis of the global market for cardiac ablation devices, which are used for the treatment of arrhythmic heart conditions, such as atrial fibrillation. The market for cardiac ablations devices is projected to see strong growth throughout the forecast period covered by this analysis, driven by demographic trends and the adoption of new technologies for the treatment of new ablation technologies, such as Pulsed Field Ablation. Devices covered within the scope of this analysis include cardiac ablation catheters. This market snapshot is intended to provide a high-level overview of the global market for cardiac ablation devices, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, insights into key market events for strategics and startups.
Companies covered in this report include: Abbott, Adagio Medical, AtriAN Medical, AtriCure, Biosense Webster, Boston Scientific, Electrophysiology Frontiers, Field Medical, Galvanize Therapeutics, Healium Medical, Johnson & Johnson, Medtronic, Stereotaxis.
Global Markets for Atrial Septal Occlusion, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


Global Markets for Atrial Septal Occlusion, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


This market snapshot from Life Science Intelligence provides an analysis of the global market for atrial septal occlusion devices. These devices are used to close atrial septal defects (ASDs), an abnormal hole in the wall of the upper chambers of the heart that are present at birth. This market snapshot is intended to provide a high-level overview of the global ASD occlusion devices market, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, insights into key market events for strategics and startups.
Companies covered in this report include: Abbott, atHeart Medical, Cardia, Hanyu Medical, Lifetech Scientific, Occlutech, W.L. Gore.
Global Markets for Aortic Grafts, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


Global Markets for Aortic Grafts, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


This market snapshot from LSI provides an analysis of the global market for aortic stent grafts for the treatment of aortic aneurysm. The market for aortic stent grafts includes open (surgical) and endovascular (transcatheter) aortic stent grafts used in the repair of abdominal aortic aneurysm (AAA) and thoracic aortic aneurysm (TAA).
This market snapshot is intended to provide a high-level overview of the global market for aortic stent grafts, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, insights into key market events for strategics and startups.
Companies covered in this report include: Cook Medical, Endoron, Endospan, Lombard Medical, Medtronic, MicroPort Scientific, Taurus Vascular, Terumo, TripleMed, W.L. Gore.
Global Markets for Interventional Cardiology Devices, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


Global Markets for Interventional Cardiology Devices, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


This market snapshot from LSI provides an analysis of the global market for interventional cardiology devices for the treatment of cardiovascular diseases such as heart failure or severe atherosclerosis. The market for interventional cardiology devices includes devices used to diagnose and treat diseases related to the coronary arteries. Devices included within the scope of this report include coronary stents, catheters, angioplasty balloons, guidewires, and intravascular ultrasound catheters. This market snapshot is intended to provide a high-level overview of the global market for interventional cardiology devices, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, insights into key market events for strategics and startups.
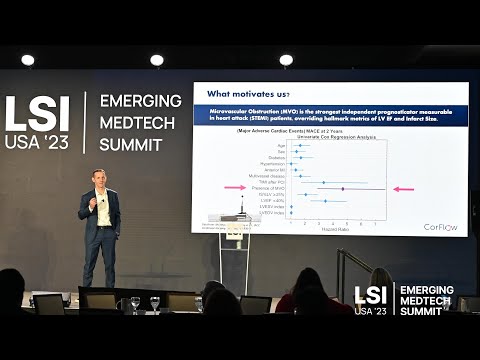
Companies covered in this report include: Abbott, Advanced Bifurcation Systems, B. Braun, BD, Boston Scientific, Cardinal Health, Cook Medical, Corflow Therapeutics, Fastwave Medical, Lemaitre Vascular, Medtronic, Merit Medical, MicroPort Scientific, NirvaMed, Philips, Teleflex, Terumo, Translumina.
Global Markets for Oncology Embolization, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


Global Markets for Oncology Embolization, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


This market snapshot from Life Science Intelligence provides an analysis of the global market for oncology embolization agents, including radioembolization, chemoembolization, and particle embolization devices. These solutions are used to occlude blood vessels to lesions and tumors to derive the target of nutrients, enhance the effectiveness of the targeted delivery of pharmaceuticals, and trigger cell death. This market snapshot is intended to provide a high-level overview of the global oncology embolization agents market, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, and insights into key market events for strategics and startups.
Companies covered in this report include: ABK Biomedical, Arsenal Medical, Boston Scientific, Cook, Cordis/Cardinal Health, Instylla, Medtronic, Merit Medical, Ned Medical, Obsidio, SirTex Medical, Stryker, Terumo, Varian.
Global Markets for Vascular Access Devices, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


Global Markets for Vascular Access Devices, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


This market snapshot from Life Science Intelligence offers an analysis of the global market for vascular access devices, which are essential for continuous and long-term access to the bloodstream for administering intravenous drugs and fluids. The market encompasses various types of devices, including peripherally inserted central catheters (PICC), midline catheters, central venous catheters (CVC), and implantable ports.
The snapshot aims to provide a comprehensive overview of the global market for vascular access devices, offering key insights into unit volumes and market forecasts from 2023 to 2028. Additionally, it includes a competitive landscape analysis of major competitors and insights into key market events for both established players and startups.
Companies covered in this report include: Access Vascular, AngioDynamics, B. Braun, Baxter, Becton, Dickinson & Company (BD), Bluegrass Vascular, C.R. Bard, Cook Medical, ICU Medical, Medline, Smiths Medical, Teleflex, Terumo, Vygon, and Yushin Medical.
Global Markets for Rotator Cuff Repair Suture Anchors, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


Global Markets for Rotator Cuff Repair Suture Anchors, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


This market snapshot from LSI provides an analysis of the global market for rotator cuff repair suture anchors for the treatment of rotator cuff injuries. The market for rotator cuff repair suture anchors includes anchors composed of biocompatible polymers and metals. This market snapshot is intended to provide a high-level overview of the global market for rotator cuff repair suture anchors, with key insights into: unit volumes from 2023 to 2028, market forecasts from 2023 to 2028, competitive landscape analysis of major competitors, and insights into key market events for strategics and startups.
Companies covered in this report include: Aevumed, Arthrex, Atreon Orthopedics, ConMed, DePuy Synthes, Embody, Inovedis, Smith & Nephew, Stryker, Tetrous, Wright Medical, Zimmer Biomet.
Global Markets for Electrical Stimulation Devices, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


Global Markets for Electrical Stimulation Devices, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


Description coming soon.
Global Markets for Wearable Monitoring Devices, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


Global Markets for Wearable Monitoring Devices, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


Description coming soon.
Global Markets for Low Complexity Medical Devices, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


Global Markets for Low Complexity Medical Devices, 2023-2028
Published: 2023
Next Update: Q4 2024
Deliverables:


Description coming soon.
Canada Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Canada Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Canada Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Canada. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Germany Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Germany Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Germany Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Germany. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
France Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


France Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The France Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for France. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
U.K. Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


U.K. Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The United Kingdom Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for the United Kingdom. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Italy Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Italy Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Italy Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Italy. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Spain Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Spain Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Spain Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Spain. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Poland Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Poland Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Poland Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Poland. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Netherlands Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Netherlands Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Netherlands Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for the Netherlands. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Belgium Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Belgium Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Belgium Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Belgium. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Sweden Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Sweden Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Sweden Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Sweden. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Switzerland Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Switzerland Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Switzerland Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Switzerland. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Denmark Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Denmark Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Denmark Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Denmark. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Finland Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Finland Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Finland Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Finland. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Norway Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Norway Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Norway Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Norway. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
China Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


China Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The China Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for China. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
India Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


India Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The India Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for India. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Japan Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Japan Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Japan Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Japan. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
South Korea Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


South Korea Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The South Korea Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for South Korea. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Australia Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Australia Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Australia Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Australia. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Thailand Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Thailand Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Thailand Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Thailand. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Malaysia Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Malaysia Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Malaysia Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Malaysia. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Singapore Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Singapore Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Singapore Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Singapore. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
New Zealand Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


New Zealand Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The New Zealand Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for New Zealand. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Caribbean Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Caribbean Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Caribbean Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for the Caribbean region. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Argentina Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Argentina Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Argentina Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Argentina. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Colombia Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Colombia Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Colombia Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Colombia. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Chile Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Chile Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Chile Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Chile. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Guatemala Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Guatemala Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Guatemala Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Guatemala. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Dominican Republic Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Dominican Republic Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Dominican Republic Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for the Dominican Republic. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Costa Rica Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Costa Rica Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Costa Rica Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Costa Rica. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Panama Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Panama Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Panama Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Panama. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Mexico Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Mexico Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Mexico Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Mexico. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Brazil Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Brazil Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Brazil Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Brazil. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Turkey Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Turkey Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Turkey Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Turkey. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
Russia Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


Russia Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The Russia Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for Russia. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.
South Africa Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


South Africa Surgical Procedure Volumes, 2018-2029
Published: 2022
Next Update: Q2 2024
Deliverables:


The South Africa Surgical Procedure Volumes Dashboard provides historical and forecasted volumes from 2018 to 2029 for South Africa. Understand trends in procedure volume adoption and growth for over 200 procedures across 12 major procedure markets (Cardio, Ortho, General Surgery, OB/GYN, Urology and more). Data is updated and expanded annually to maintain a consistent pulse on the procedure utilization to empower strategic decisions on commercialization and new product development.