Overview
Valued at ~$3.07 billion in 2023, the cardiac ablation devices market is projected to reach
~$5.42 billion by 2028, increasing at a CAGR of 12.0% over the 2023-2028
forecast period. This Market Snapshot is part of LSI’s Market Intelligence platform, your
one-stop-shop for global medtech market sizing and analysis, procedure volume data, startup company- and
deal-tracking, curated insights, and more.
Atrial fibrillation (AFib), atrial flutter, and atrial tachycardia are rapid heartbeat symptoms that can be
treated by ablation technology. Catheter ablation has emerged as an effective approach to treat AFib. Ablation
is a complex procedure requiring sophisticated diagnostic equipment to map the heart’s electrical activity to
identify areas of the heart causing irregular electrical activity. Radiofrequency (RF), cryoablation, and
pulsed-field ablation (PFA) are types of energy used to create lesions within the heart to disrupt electrical
activity that causes an irregular heart.
Products included within the scope of this analysis include:
Cardiac ablation catheters
This Market Snapshot is intended to provide a high-level overview of the global market for cardiac ablation
devices, with key insights into:
Unit volumes from 2023 to 2028
Market forecasts from 2023 to 2028
Market insights
Competitive landscape analysis of major competitors
Insights into key market events for strategic and startups
Cardiac Ablation Devices Market Snapshot Summary
| Snapshot Aspect | Data and Details |
| Base Year for Estimate | 2023 |
| Forecast Period | 2023 - 2028 |
| Market Size in 2023 | $3.07 billion |
| CAGR | 12.0% |
| Projected Market Size in 2028 | $5.42 billion |
Cardiac Ablation Devices Market Insights
The cardiac ablation devices market is experiencing strong growth, driven by advancements in screening,
diagnosis, and treatment technology for atrial fibrillation (AFib), alongside increased awareness of the
condition. With an estimated 40 to 60 million people living with AFib worldwide, the number of cardiac ablation
procedures is expected to continue rising, particularly as improved detection methods, such as ambulatory
monitoring devices, gain wider use. Technological advancements—including the introduction of force-sensing
catheters and PFA technology—are further fueling market expansion by offering more precise treatments and better
long-term outcomes, such as reduced recurrence of AFib.
The market is expected to continue growing at a strong pace, outpacing the overall medical device market, driven
by the increasing shift from lifelong drug treatments to ablation therapies. As more patients are diagnosed with
AFib and new, more effective ablation technologies are introduced, the demand for cardiac ablation devices will
remain robust, positioning the market for sustained growth in the coming years.
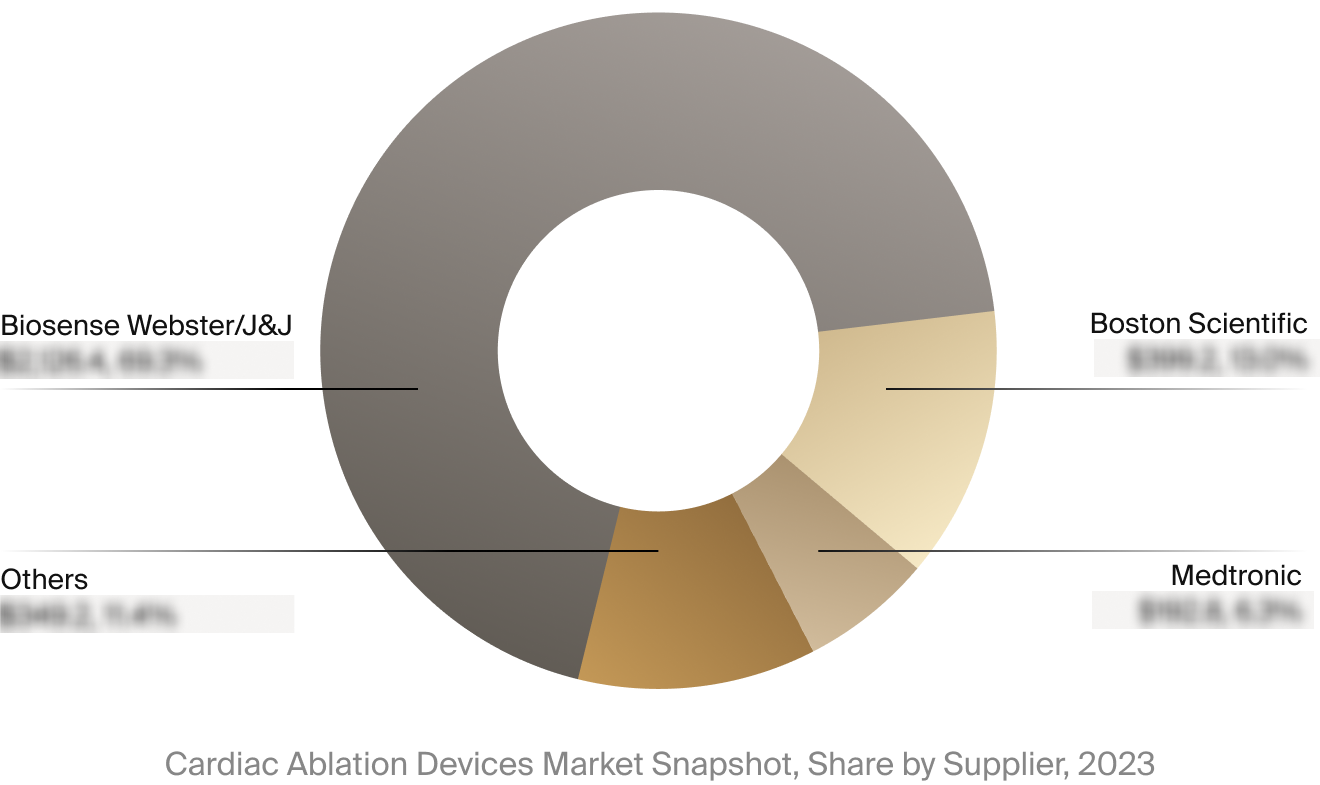
Competitive Landscape
The full Market Snapshot includes a robust analysis of the competitive landscape for the cardiac ablation
devices market. This includes estimated market revenue and market share for key players, such as Biosense
Webster/Johnson & Johnson, Boston Scientific, and Medtronic.
Select Market Events
| Company | Date | Type | Event |
|
Biosense Webster
|
3/2024 | Regulatory Filing | Biosense Webster announced that the company has applied for FDA Premarket Approval of the VARIPULSE Platform. The company received the CE Mark for VARIPULSE in February 2024. |
|
Boston Scientific
|
1/2024 | Regulatory Approval | Boston Scientific received FDA approval for the FARAPULSE PFA System. |
Key Companies Covered
Abbott
Affera
AliveCor
AtriCure
Biosense Webster
Boston Scientific
CardioFocus
Field Medica
Galvanize Therapeutics
Johnson & Johnson
Medtronic
Stereotaxis